
The importance of the mesentery in emergency general surgery: ignore the mesentery at your peril
Introduction
The mesentery is largely ignored in discussions of emergency abdominal surgery. This omission arises from an erroneous understanding of mesenteric anatomy. According to the model described by Henry Gray in 1858, and uncontested until recently, there are multiple mesenteries (1). Each mesentery was described as attaching to the posterior abdominal, thereby giving rise to a particular peritoneal landscape in the abdominal cavity. A mesentery that is present at some levels of the intestine, and absent at others, would be dauntingly complex and variable from an anatomical perspective. In turn these properties would mean it is highly unlikely to play a major role in commonplace abdominal emergencies.
The mesentery is now accepted as a single, substantive and continuous entity with properties supporting its description as an organ (1-3). The new mesenteric model provides an anatomical frame of reference that explains the aetiology and pathology of multiple surgical emergencies of the abdominal cavity. As a result, the current mesenteric frame of reference explains the clinical manifestation of many abdominal conditions (4,5). Mesenteric continuity simplifies and enhances the radiological interpretation of the abdomen with major implications at diagnostic and interventional levels (6). The adoption of mesenteric-based surgical treatment strategies greatly enhances the surgeon’s ability to treat emergencies of the abdomen.
The following article will demonstrate how recent clarification of mesenteric anatomy and the demonstration of continuity profoundly alter our perspective on surgical emergencies of the abdomen. It will demonstrate how the perspective afforded to us by the new mesenteric model greatly enhances our diagnostic and therapeutic capabilities.
History: why the mesentery came to be largely ignored
Mesenteric factors have received little focus in descriptions of surgical emergencies of the abdomen, in a tradition that can be traced back to the early 19th century surgery (7). Distinguished surgeons of the time [including Amussat (France) and Callisen (Denmark)] described lumbar colotomy in treatment of an obstructing distal intestinal lesion (8,9). Importantly, their descriptions emphasised that on the left side of the colon, the left mesocolon was always absent (unless one were to artificially develop one through traction) (9). It is likely that anatomical assertions of the surgical community influenced Henry Gray (a surgeon) when he described insertion (or attachment) of the small intestinal mesentery to the posterior abdominal wall. This insertion (or attachment) became known as the root of the mesentery. The following is Gray’s original statement:
“Its (i.e., the mesentery) root, the part connected with the vertebral column, is narrow, about six inches in length, and directed obliquely from the left side of the second lumbar vertebra, to the right sacro-iliac symphysis.” (10).
Insertion or attachment of the mesentery implied the small intestinal mesentery was not continuous with the right mesocolon. Gray’s short description of the “mesenteries” placed the right and left mesocolon posterior to their respective regions of intestine (i.e., not connected with the small intestinal region of mesentery). Whilst Gray was a surgeon, his anatomical textbook became and remains the premier reference text in human anatomy (11).
The concept of multiple separate mesenteries gained further support by another prominent surgeon, Sir Frederick Treves [1888] (12). Treves described how the left and right mesocolon were largely absent in the majority of the cadavers he examined and as such their presence could only be expected in a minority of cases. Following on from his assertions, occurrence of mesentery attached to the right and left colon was subsequently was routinely described as “anomalous” leading to development of volvulus in these regions (7,12-14). These concepts were indoctrinated in mainstream anatomical literature as reflected in the following statement by Cunningham:
“…the mesenteries occasionally found in connection with the ascending and descending portions of the colon….” (from Cunningham’s Manual of Practical Anatomy, second edition, 1896) (15).
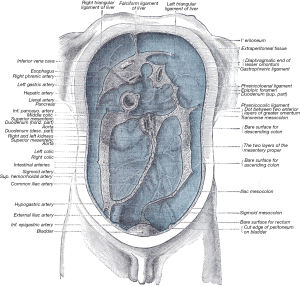
Cunningham’s manual became and remains the premier guide for students conducting cadaveric dissections. The adoption of Gray’s description was aided by development of images such as that of Auguste Sheridan Delepine (Figure 1). Delepine’s image visually explained Gray’s assertions and Treve’s findings and was routinely used as the starting point of descriptions of abdominal anatomy for the following century.
The status quo persisted until recently when we demonstrated the right and left mesocolon are always present in the adult human. More importantly, we demonstrated that the small intestinal region of mesentery is always continuous with the right mesocolon (16). This led to the description of mesenteric continuity from the duodenojejunal flexure to anorectal junction. Follow-on studies demonstrated continuity from the oesophagogastric to anorectal junction (1). A minor but important edit appeared in the 41st edition of Gray’s Anatomy;
“The mesocolon extends along the entire length of the colon and is continuous with the small intestinal mesentery proximally and the mesorectum distally.” (11).
This minor edit has major implications. Firstly, it corrects the error originally introduced by Henry Gray (see above). Secondly, it compels the scientific and clinical communities to now reappraise mesenteric (and related peritoneal) anatomy. Such a reappraisal will demonstrate that the mesentery is (I) continuous; (II) a primary determinant of peritoneal anatomy; and (III) the organ around which all abdominal digestive organs are positioned (17).
The importance of the mesentery in diagnosis and management of appendicitis
Appendicitis is the most common abdominal surgical emergency (18). Challenges continue to arise in relation to clinical diagnosis and clinicians are often criticised for an apparent delay in diagnosis (19,20). Challenges also arise intraoperatively, when trainees and indeed experienced surgeons can sometimes struggle to localise and mobilise the appendix for removal (21). Many of these difficulties are explained by the anatomy of the mesoappendix.
The position of the appendix is determined by (I) the location of the appendix base; and (II) the mesoappendix. The mesoappendix is a mesenteric appendage containing appendiceal vessels. Literature on anatomy of the meso-appendix is sparse, a point that is not surprising given the classic model of discontinuity.
Given (I) the mesentery is continuous, and (II) the small intestinal region of mesentery continues as the right mesocolon, a mesenteric apex occurs at the ileocaecal region (16,22). This has been arbitrarily termed the ileocaecal mesenteric confluence. The mesoappendix always arises from the posterior surface of this confluence, near the ileocaecal junction.
The abdominal digestive system comprises organs positioned around a central mesenteric frame. Once the abdominal digestive system has adopted a final conformation during development, it is maintained in this conformation through three major mechanisms. These are (I) vascular points of connection; (II) mesenteric regions of attachment to the posterior abdominal wall; and (III) development of the peritoneal reflection (1,5,17). The right and left mesocolon, medial region of the mesosigmoid, and the entire mesorectum, are attached. As the right mesocolon attaches to the posterior abdominal wall, and the meso-appendix arises from its posterior surface, it not difficult to understand how the appendix often has a retro-caecal position (23). In turn, this means appendiceal inflammation can occur with little clinical symptomatic manifestation other than a vague sense of discomfort in the right iliac fossa (24). The origin of the mesoappendix and the process of attachment of the mesentery to the posterior abdominal wall mean that appendicitis should never be considered a straightforward diagnosis but rather one that continues to challenge.
The surgical treatment of appendicitis can also be challenging, for the same anatomical reasons. Anatomical and surgical texts usually depict the relationship between the appendix and the caecum in an overly simplistic and schematic manner (11,25,26). The reality in practice is that the surgeon can explore the ileocaecal region for a considerable length of time, ever hopeful that he or she will eventually identify a tubular structure that resembles the appendix. As a result, reference textbook depictions do not reflect commonplace intraoperative experience, where the surgical trainee (and sometimes even the experienced surgeon), have difficulty in localising the retro-caecal appendix (27,28)). If the appendix is operatively approached according to a mesenteric-based strategy, appendectomy can be standardised in even the most difficult of cases. Accordingly, the ileocaecal peritoneal reflection must first be divided and the embryological process of mesenteric attachment reversed, by detaching the ileocaecal mesentery from the posterior abdominal wall. In this manner the retrocaecal appendix will almost always become immediately apparent.
The mesenteric-based approach also helps in cases of non-rotation (see below) where the mesentery has an alternative conformation and the meso-appendix takes up a highly variable position in the abdomen. In non-rotation, the ileocaecal mesenteric confluence is centrally positioned, or can lie in the left upper quadrant. The meso-appendix is similarly positioned and appendiceal inflammation results in symptoms in the epigastrium or left upper quadrant, rather than in the right iliac fossa (29).
The mesentery in formation of volvulus
During embryological development, the intestine arises at the periphery of the mesentery and receives connective tissue and cellular inputs from the mesentery. This relationship is maintained into adulthood when the position of the intestine, and its conformation, are determined by the mesentery. The small intestinal mesentery is normally centrally positioned in the abdomen, with the right mesocolon positioned on the right, and the left mesocolon positioned on the left. The result is that the small and large intestine take up an overall spiral conformation (30-34).
As mentioned above, mesenteric attachment must occur in order to anchor the mesentery (and by definition the intestine) in position. Where the small intestinal region of mesentery continues as the right mesocolon it becomes attached to the posterior abdominal wall. The flexures are regions where the mesentery changes from attached to non-attached along its radial axis (1,5,16,17,35,36).
The mesosigmoid resembles the small intestinal and right mesocolic regions of the mesentery. The medial region of the mesosigmoid is attached (thus resembling the right mesocolon) whilst the lateral region is non-attached (resembling the small intestinal region of mesentery). The differential between attached and non-attached regions of mesentery is an important concept and a determinant of the development of volvulus. In keeping with this, regions of the intestine where such a differential can be pathological (i.e., the ileocaecal and sigmoidal regions) are most prone to the development of volvulus (1,5,16,17,35,36).
Volvulus is a common abdominal emergency that has been described mainly in terms of a twisting of the intestine (37). With the exception of ileocaecal volvulus, descriptions largely ignored the mesentery. Even in the case of ileocaecal volvulus the literature was based on the erroneous concept of mesenteric fragmentation (38). Most depictions of ileocaecal volvulus attribute its development to the persistence of an anomalous ileocaecal mesentery (22,39).
Considering the position of the intestine is determined by the mesentery, one sees that volvulus arises where more mesentery is non-attached than is attached. In this setting, the non-attached mesentery (and by definition the intestine) twists around a mesenteric pedicle (40). The base of the pedicle corresponds to the region where the mesentery is attached. Perhaps the most drastic and catastrophic example of volvulus arises in the setting on non-rotation (see below) (41-44). Here, non-attachment of the mesentery means that it is free to rotate around the root region containing the super mesenteric artery and vein (45,46). In adulthood, volvulus occurs mainly at sigmoidal and ileocaecal levels. Sigmoid volvulus is explained by the differential in length between the attached, medial region of the mesosigmoid, and the non-attached, lateral region of the mesosigmoid (47-49). In ileocaecal volvulus, attachment of the right mesocolon is inadequate, and volvulus again occurs around the region of mesentery that is attached (50,51).
The role of the mesentery in mal or non-rotation
One of the commonest abdominal emergencies in the first year of life is called mal or non-rotation (52-54). This is generally described as a failure of rotation of the mesentery with the result that the small intestine is positioned to the right, the right colon in the centre and the left colon to the left of the abdominal cavity (55). According to the classic model of development, the mesentery is described as “rotating” around the superior mesenteric axis, until the intestine comes to adopt the conformation normally seen in the adult (see above). The positioning of the small intestine to the right and the right colon in the middle was attributed to failure or incomplete rotation, and hence called “mal” rotation (54,56-59).
The current model of mesenteric development describes development of the intestinal tube at the periphery of the mesentery. At the intestinal margin, the mesentery coils in tandem with the intestine. Primary and secondary coils arise as the hindgut and associated mesentery take up and become anchored to the left of the midline. At week 23, the forerunner of the right colon and its associated mesentery are positioned in the epigastrium. Elongation of both, coupled with development of the liver cause the right colon and mesentery to take up a position to the right, and displace the small intestine and mesentery into the mid-region. Importantly, the connection between the developing mesentery and the posterior abdominal wall does not rotate around the superior mesenteric artery. The concept of rotation developed through observations of coiling. If a developing coil is observed from a particular viewpoint, any point on the coil appears to “rotate” around a central point (30,54).
In mal or non-rotation intestino-mesenteric development stops at the point when the right colon and its contiguous mesentery are centrally located. This means the small intestine and contiguous mesentery remain on the right side of the abdomen. Importantly, the mesentery is still continuous, and has an overall folded or accordion-like conformation. This in itself is not pathogenic, as reflected in the fact that most cases of mal or non-rotation are diagnosed incidentally in patients undergoing surgery for an alternative reason (42,60,61). A pathological scenario arises when the mesentery does not attach adequately to the posterior abdominal wall. In this setting, the “mal-rotated” mesentery is free to twist around the superior mesenteric artery pedicle. The resultant rotation around the superior mesenteric artery is catastrophic, occluding the artery and leading to widespread mid and hindgut necrosis (42,54,62).
Mal or non-rotation is thus a primary mesenteropathy, an abnormality arising primarily of the mesentery and abnormal attachment of this. When considered in terms of its mesenteric basis, the diagnosis and treatment are greatly simplified. Treatment involves recapitulating normal embryological events including (I) placing the right colon and mesentery in in the right flank; (II) displacing the small bowel and mesentery towards the centre; and (III) attaching the right mesocolon to the posterior abdominal wall (54).
The role of the mesentery in diagnosis and treatment of emergencies in Crohn’s disease
In Crohn’s disease, surgery is required for two indications: (I) failure of medical therapy to control symptoms; and (II) development of complications. Complications are usually surgical emergencies and include intestinal obstruction, perforation, fistulation or abscess formation (63). Although the overall incidence of Crohn’s disease is low, the cumulative impact of the related emergencies exerts a considerable personal and societal toll (64,65). Up until recently, the mesentery has received relatively little attention in regards to the diagnosis and treatment of Crohn’s related abdominal emergencies (66).
As with other surgical emergencies of the abdomen, mesenteric factors are highly significant in the diagnosis and treatment of Crohn’s disease. The diagnosis of Crohn’s disease continues to be challenging in the absence of a specific biomarker (67). At present, the only hallmark of the disease that is pathognomonic, and thus could serve as a diagnostic tool, is fat wrapping (also called creeping fat) (68). This is where the mesentery advances over the surface of contiguous intestine (69). In severe cases, the intestinal surface can be almost entirely obscured from view (Figure 2). Fat wrapping is difficult to detect radiologically (70). Studies are ongoing to determine radiological correlates of fat wrapping in early, intermediate and advanced states. For now, a definitive diagnosis of Crohn’s disease continues to rely on identification of mesenteric, transmural and mucosal abnormalities in tandem, in surgical specimens.
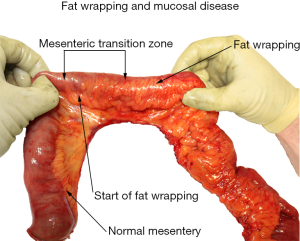
Although mesenteric factors are of extreme importance in the surgical treatment of Crohn’s, they have received little attention and the focus has ritually remained on the intestine. Again, this is not surprising and can be partially explained by the fact that a discontinuous structure (i.e., mesentery) is unlikely to play a major role in a condition that can involve any region of the continuous intestinal tube. The identification of mesenteric continuity now enables us to challenge that notion (66).
The convention in Crohn’s disease is to amputate the mesentery as close to the intestine as possible. This convention arose from a technical conservatism related to the amount of intestine excised, but also from concerns regarding the possibility of haemorrhage from the diseased mesentery. In Crohn’s disease, the mesentery can be thickened, oedematous, fibrotic and highly vascular. Alternatively, it can be oedematous and remarkably soft in consistency. As a result, it is prone to haemorrhage and this in turn is often difficult to control. Inflamed mesenteric surfaces adhere to each other and to other organs. This property often leads to the development of a phlegmon, which if untangled from a mesenteric perspective can bleed extensively. In addition, the mesentery is often involved in walling off an abscess cavity. Mesenteric factors combine to make surgery in Crohn’s disease technically demanding (4,71).
Clarification of mesenteric anatomy, and development of haemostatic mechanisms of division of the mesentery, have converged to permit inclusion of the mesentery as part of surgical resections in Crohn’s disease. Early data indicate this may be associated with improved long-term outcomes, without compromising on short term operative outcomes (71). These observations are currently the focus of a number of international randomised controlled trials.
The mesentery can also be exploited in additional manners in Crohn’s disease. For example, mesenteric manifestations of disease commence gradually at the mesenteric transition zone at which mural and mucosal disease develop in tandem. As a result, placement of the intestinal margin proximal to the transition zone appears to provide the surgeon with a reliable means in deciding where to divide the intestine (66,71). This is welcome given these patients often require repeat surgery with the implication that intestinal conservation is important. In addition, the severity of fat wrapping around the intestinal surface also appears to be an important determinant of recurrence (4,66,71-73). Identification of advanced fat wrapping may help select patients at increased risk of recurrence and who may benefit from adjuvant treatment strategies.
Mesenteric factors in re-operative emergency abdominal surgery
Following colorectal surgery, the incidence of short term complications ranges from 30% to 50% (74). Many patients will require re-operation as part of management of a haematoma, abscess, anastomotic leak, or intestinal obstruction (75,76). Re-operative surgery is challenging due to intestinal and mesenteric factors. Not surprisingly, the later have received little consideration to date (77).
Firstly, the mesentery provides a vast surface area along which adhesions can arise. As a result, regions of mesentery can adhere to other organs, or to other regions of mesentery (78). This is an important and frequently overlooked consideration in the postoperative period. After day ten postoperatively, intra-mesenteric adhesions (i.e., adhesions between different regions of the mesentery) are highly vascular and densely fibrotic (79). Division of these is fraught with difficulty and danger, as it may lead to de-mesothelialisation of an organ (including the mesentery). De-mesothelialisation of the mesentery exposes underlying mesenteric vessels which can become compromised. In turn this sometimes leads to vascular insufficiency in contiguous intestine. Bleeding from mesenteric vessels is problematic and difficult to control. These vessels tend to loose blood in a gradual rather dramatic manner. This persistent (albeit low volume) blood loss obscures intra-mesenteric planes of dissection (thus placing the mesentery at further risk). As with most forms of adhesion, inter-mesenteric adhesions change consistency to become softer and more amenable to division, in the first three months following surgery (77,80). The process whereby adhesions can “soften” can be slowed by the presence of persistent inflammation and infection.
In the re-operative setting, it is essential the surgeon has an accurate understanding of mesenteric anatomy in general. He/she needs to be able to differentiate mesenteric from other types of fat. These include retro-peritoneal, omental, supra-vesicular and subcutaneous fat. The bladder dome is located immediately beneath the supra-vesicular fat pad. The ureter, gonadal vessels and kidneys are located within retroperitoneal fat. If the surgeon is unaware of the differences between these and mesenteric fat, the risk to contained structures is considerable. The surgeon must be able to differentiate different regions of mesentery. It is not uncommon for mesocolic regions to adhere to small intestinal mesenteric regions, and if the surgeon is not aware of the presence of both, then he/she may inadvertently damage either (77).
In the emergency postoperative setting, the surgeon is often required to mobilise the intestine for defunctioning or resection. The mobility of the intestine is determined by that of the mesentery. When there is significant intraperitoneal sepsis, the mesentery can be oedematous, fore-shortened and thus difficult to mobilise. This, in turn, translates to difficulty in mobilising contiguous intestine. The surgeon must anticipate this mesenteric-based difficulty and have strategies to overcome mesenteric-based limitations on intestinal mobilisation.
Before re-entering an abdomen previously operated by another surgeon, it is important to consider whether the mesentery been fully or partially resected in the previous resection. The presence of residual mesentery is important for reasons detailed above. In addition, the embryological plane between residual mesentery and underlying fascia, is always more difficult to excavate, than it in the uninterrupted state. In the re-operative setting the plane between mesentery and underlying fascia resembles that encountered when there has been inflammation, sepsis and exposure to radiation. The fascia is more vascular and densely adherent to the under surface of the mesentery. Their separation is always associated with more blood loss than normally expected (77).
Mesenteric factors in the surgical management of advanced intra-abdominal disease
The surgical management of advanced disease always poses numerous challenges. One of the greatest of these lies in the anatomical involvement of multiple structures including other organs and the abdominal wall. Advanced disease requiring surgical management mostly occurs in the setting of perforated appendicitis, Crohn’s, diverticular disease and locally advanced malignancy.
A key tenet in surgical treatment of advanced disease is to isolate the pathology as much as is possible from an anatomical perspective. This concept should be illustrated with a common example. In perforated diverticular disease, the dome of the bladder may be adherent to the colon. In addition, inflammatory changes may transgress normal embryological planes into the retroperitoneum, where the ureter and other structures can be secondarily involved. This is not an uncommon pattern as it also seen in advanced Crohn’s colitis, perforated ulcerative colitis, and in advanced rectosigmoid malignancy. Isolation of the pathology involves mobilisation of surrounding structures along embryological planes, until the pathology is circumferentially exposed. In doing this, the extensive nature of the pathology can be readily characterised with the result that blood-loss and trauma to adjacent organs can be carefully controlled.
The mesentery is contiguous with all abdominal digestive organs. In addition, the mesentery maintains the position of all abdominal digestive organs (1,5). As a result, isolation of an advanced intra-abdominal pathology almost always requires detachment of the nearby mesentery. One can take the scenario described in the preceding paragraph as a representative example. In order to isolate the pathology involved, the intestine must be divided proximal and distal to it. Next, the mesosigmoid must be divided. Only then is the pathology fully and circumferentially isolated. Neither intestinal nor mesenteric division can be safely achieved without first detaching the mesentery (81,82).
In turn, mesenteric detachment requires that the surgeon understand the embryological mechanisms that led to attachment in the first instance (i.e., formation of the fascia and peritoneum). It is only by understanding the mechanisms of attachment (and how these are surgically disrupted) that one will be able to adequately isolate advanced intra-abdominal pathology in order to control blood loss as well as damage to nearby structures (81,82).
The mesentery during stoma formation for obstructing intestinal lesions
Intestinal obstruction secondary to malignancy is not an uncommon surgical emergency. In many instances, a defunctioning ostomy is used either as a final treatment, or in managing the patient prior to definitive surgery. Stoma formation is classically regarded as a simple procedure. In most texts describing the technique, mesenteric factors are given little emphasis. Not surprisingly, the operation is often allocated to trainees who struggle with the result that the patient suffers considerable morbidity due to a poorly constructed stoma (83).
Transverse colostomy formation is often used in the treatment of an obstructing colonic or rectal lesion. Transverse colostomy formation is often assigned to the surgical trainee as it is usually anticipated that little technical difficulty will be encountered. This is predicated on the concept that the transverse mesocolon is mobile, and that the transverse colon will “practically offer itself” for the trainee to manage. When one considers the operation is mesenteric terms, one anticipates an entirely different set of challenges that almost always occur, albeit with varying patterns.
As mentioned above, the mesentery is continuous. This means the hepatic flexure comprises a mesenteric component (among others) where the right mesocolon detaches and continues as the transverse mesocolon. The attachment of the mesenteric component of the hepatic flexure is an important determinant of mesocolic (and hence colonic) mobility. If the mesenteric component of the flexure is densely adherent and anatomical remote (as it often is in obese individuals), then difficulty will be encountered in mobilising the proximal transverse colon for colostomy formation (17,35,36,83).
The next important factor for consideration is the greater omentum. This is always adherent to the cephalad surface of the transverse mesocolon. The adhesion of both is often considerable, and presents a further challenge to delivery of the colon into a wound. In addition, the plane between omentum and mesocolon is obscured from direct view by the reflection of peritoneum that always occurs between the greater omentum and the transverse colon. This must be divided in order to expose the plane of adherence between the greater omentum and the upper surface of the transverse mesocolon (17,35,36,83).
Other mesenteric factors are of considerable importance in stoma formation. As mentioned above, intestinal mobility is determined by mesenteric mobility. For example, in laparoscopic loop ileostomy formation, it is important there is no tension on the intestine (84). However, it is essential there is no tension on contiguous mesentery. Mesenteric tension (rather than intestinal tension) will cause the stoma to retract and lead to considerable morbidity for the patient.
The mesentery in radiological assessment of abdominal emergencies
Abdominal radiologists continue to base their appraisal of mesenteric anatomy on the classic model. Not surprisingly they frequently commence descriptions of this topic with the statement that it is complex (85). In addition, some have directly commented that the appearance of the mesentery on computerised tomographic imaging of the abdomen, is difficult to reconcile with prevailing (i.e., classical) models of abdominal anatomy (86,87). The traditional (peritoneal-based) approach to the interpretation of abdominal radiological imaging was founded on the classic model of mesenteric anatomy. In keeping with this, the classic model of peritoneal reflections (and hence spaces) was also flawed. For example, the classic model holds that certain regions of the intestine were intraperitoneal, whilst others were retroperitoneal. Not surprisingly, this categorisation of the location of abdominal digestive organs hampered diagnostic and surgical approaches.
According to the mesenteric-based model of abdominal anatomy, the abdomen is divisible into mesenteric and non-mesenteric domains. The mesenteric domain comprises all abdominal digestive organs, centred on the mesenteric frame. The latter also contains all digestive organ vascular, neuronal and lymphatic circuitry. The non-mesenteric domain lies posterior to Toldt’s fascia and includes the kidneys, ureters, inferior vena cava and aorta. This model is anatomically correct and easier to use in the interpretation of abdominal radiology, than is the conventional peritoneal-based model (6,85).
Several benefits to the mesenteric-based approach to modelling of the abdominal cavity, quickly become apparent. For example, the entirety of all abdominal digestive organs is located within the mesenteric domain (1,5,17). One no longer has to reconcile how some regions of the digestive tract are intraperitoneal, whilst others are retroperitoneal. All lie entirely in the mesenteric domain. Another benefit lies in explaining patterns of lymphatic spread. Heretofore, it has not been possible to anatomically rationalise how right colon cancer can metastasize to gastroepiploic lymph nodes, or how rectal cancers could metastasise to nodes in the portal pedicle. This pattern of spread is immediately explained by the concept of mesenteric continuity (88). A further benefit to the mesenteric-based model lies in the fact that it explains the pattern of spread of fluid collections in pancreatitis. These often extend from the pancreatic region, tracking under the left mesocolon, detaching the later from the posterior abdominal wall. This phenomenon is also readily explained by the concept of mesenteric continuity, and subdivision of the abdominal cavity into mesenteric and non-mesenteric domains.
Future directions
According to the current model of mesenteric anatomy, the mesentery is continuous and maintains all abdominal digestive organs in position and in continuity with other systems. In turn, the mesentery is held in position by attachment to Toldt’s fascia, and formation of the peritoneal reflection. This means that mesenteric anatomy is a determinant of peritoneal anatomy (and not vice versa). The mesenteric-based model means the abdomen can be subdivided into mesenteric and non-mesenteric domains, with considerable implications for diagnosis and treatment of emergency conditions of the abdomen.
Several intriguing opportunities arise in relation to abdominal symptoms in general, their investigation and management. As the mesentery was poorly understood until recently, so too was the importance of mesenteric attachment. In fact, this factor has been largely overlooked when it comes to abdominal pain. It is feasible it may prove important in conditions for which a mechanical cause has not been identifiable. For example, the label “irritable bowel disease” is given to a circumstance in which all investigative modalities available have not identified an underlying abnormality. Yet, patients with irritable bowel syndrome have real symptoms. Similar examples include recurrent abdominal pain, abdominal migraine and infantile colic, in the paediatric population. Here again, the symptoms are actual, yet an underlying abnormality is not apparent when currently available investigative modalities are employed. Defects in mesenteric attachment are not apparent using current imaging modalities. Notwithstanding this, they can lead to catastrophic consequences as described above. It is feasible that minor degrees of attachment-related defects are present at a far more common rate than previously thought, and that these could provide a much sought after mechanical basis for many abdominal symptoms. It is enticing to think that irritable bowel syndrome can in actual fact be rationalised according to an anatomical foundation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Coffey JC, O'Leary DP. The mesentery: structure, function, and role in disease. Lancet Gastroenterol Hepatol 2016;1:238-47. [Crossref] [PubMed]
- Argikar AA, Argikar UA. The mesentery: an ADME perspective on a 'new' organ. Drug Metab Rev 2018.1-8. [Crossref] [PubMed]
- Neumann PE. Another new organ! is this a golden age of discovery in anatomy? Clin Anat 2018;31:648-9. [Crossref] [PubMed]
- Rivera ED, Coffey JC, Walsh D, et al. The Mesentery, Systemic Inflammation, and Crohn's Disease. Inflamm Bowel Dis 2018. [Epub ahead of print]. [Crossref] [PubMed]
- O'Leary DP, Peirce C, Coffey JC. Response: "Prophylactic Negative Pressure Dressing Use in Closed Laparotomy Wounds Following Abdominal Operations: What We Really Know? Ann Surg 2018;268:e20-1. [PubMed]
- Coffey JC, Culligan K, Walsh LG, et al. An appraisal of the computed axial tomographic appearance of the human mesentery based on mesenteric contiguity from the duodenojejunal flexure to the mesorectal level. Eur Radiol 2016;26:714-21. [Crossref] [PubMed]
- Sehgal R, Coffey JC. Historical development of mesenteric anatomy provides a universally applicable anatomic paradigm for complete/total mesocolic excision. Gastroenterol Rep (Oxf) 2014;2:245-50. [Crossref] [PubMed]
- Amussat JZ. Mémoire sur la possibilité d'établir un anus artificiel dans la région lombaire sans pénétrer dans le péritoine. Germer-Baillière, 1839.
- Tilson D. The origins and evolution of colostomy. BJS 1934;22:142-54. [Crossref]
- Gray H. Anatomy--descriptive and surgical. J.W. Parker and Son, 1858.
- Standring S. Gray's Anatomy: The Anatomical Basis of Clinical Practice. Elsevier Limited, 2016.
- Treves F. Lectures on the Anatomy of the Intestinal Canal and Peritoneum in Man. Br Med J 1885;1:580-3. [Crossref] [PubMed]
- Coffey JC. Surgical anatomy and anatomic surgery - Clinical and scientific mutualism. Surgeon 2013;11:177-82. [Crossref] [PubMed]
- Coffey JC, Sehgal R, Walsh D. History. Mesenteric Principles of Gastrointestinal Surgery: Basic and Applied Science. CRC Press, 2017:3-11.
- Cunningham DJ. Manual of Practical Anatomy. vol v. 1. Young J. Pentland, 1896.
- Culligan K, Coffey JC, Kiran RP, et al. The mesocolon: a prospective observational study. Colorectal Dis 2012;14:421-8; discussion 428-30. [Crossref] [PubMed]
- Coffey JC, Dillon M, Sehgal R, et al. Mesenteric-Based Surgery Exploits Gastrointestinal, Peritoneal, Mesenteric and Fascial Continuity from Duodenojejunal Flexure to the Anorectal Junction--A Review. Dig Surg 2015;32:291-300. [Crossref] [PubMed]
- Sartelli M, Baiocchi GL, Di Saverio S, et al. Prospective Observational Study on acute Appendicitis Worldwide (POSAW). World J Emerg Surg 2018;13:19. [Crossref] [PubMed]
- Petroianu A. Diagnosis of acute appendicitis. Int J Surg 2012;10:115-9. [Crossref] [PubMed]
- Omundsen M, Dennett E. Delay to appendicectomy and associated morbidity: a retrospective review. ANZ J Surg 2006;76:153-5. [Crossref] [PubMed]
- Deshmukh S, Verde F, Johnson PT, et al. Anatomical variants and pathologies of the vermix. Emerg Radiol 2014;21:543-52. [Crossref] [PubMed]
- Coffey JC, Sehgal R, Walsh D. Pathology of the mesentery. Mesenteric Principles of Gastrointestinal Surgery: Basic and Applied Science. CRC Press, 2017:85-109.
- Oruc M, Muminagic S, Denjalic A, et al. Retrocaecal appendix position--findings during the clasic appendectomy. Med Arch 2012;66:190-3. [Crossref] [PubMed]
- Baird DLH, Simillis C, Kontovounisios C, et al. Acute appendicitis. BMJ 2017.357. [PubMed]
- Trelease RB. Netter's Surgical Anatomy Review P.R.N. Elsevier. 2016.
- Williams N, O'Connell PR. Bailey & Love's Short Practice of Surgery 26E. CRC Press, 2013.
- Bencini L, Bernini M, Martini F, et al. Laparoscopic Appendectomy Performed by Residents and Experienced Surgeons. JSLS 2009;13:391-7. [PubMed]
- Siam B, Al-Kurd A, Simanovsky N, et al. Comparison of Appendectomy Outcomes Between Senior General Surgeons and General Surgery Residents. JAMA Surg 2017;152:679-85. [Crossref] [PubMed]
- Birnbaum DJ, Geffroy Y, Goin G, et al. Left Side Appendicitis with Midgut Malrotation in an Adult. J Surg Tech Case Rep 2013;5:38-40. [Crossref] [PubMed]
- Coffey JC, Sehgal R, Walsh D. Embryologic development of the mesentery, peritoneal reflection, and Toldt's fascia. Mesenteric Principles of Gastrointestinal Surgery: Basic and Applied Science. CRC Press, 2017:41-7.
- Gamblin TC, Stephens RE Jr, Johnson RK, et al. Adult malrotation: a case report and review of the literature. Curr Surg 2003;60:517-20. [Crossref] [PubMed]
- Berdon WE, Baker DH, Bull S, et al. Midgut malrotation and volvulus. Which films ar most helpful? Radiology 1970;96:375-84. [Crossref] [PubMed]
- Malek MM, Burd RS. The optimal management of malrotation diagnosed after infancy: a decision analysis. Am J Surg 2006;191:45-51. [Crossref] [PubMed]
- Kapadia MR. Volvulus of the Small Bowel and Colon. Clin Colon Rectal Surg 2017;30:40-5. [PubMed]
- Coffey JC, Sehgal R, Walsh D. Mesenteric and peritoneal anatomy. Mesenteric Principles of Gastrointestinal Surgery: Basic and Applied Science. CRC Press, 2017:11-41.
- Coffey JC, Sehgal R, Walsh D. Mesenteric component of flexure mobilization. Mesenteric Principles of Gastrointestinal Surgery: Basic and Applied Science. CRC Press, 2017:293-301.
- Lou Z, Yu ED, Zhang W, et al. Appropriate treatment of acute sigmoid volvulus in the emergency setting. World J Gastroenterol 2013;19:4979-83. [Crossref] [PubMed]
- Santín-Rivero J, Núñez-García E, Aguirre-García M, et al. Intestinal volvulus. Case report and a literature review. Cir Cir 2015;83:522-6. [PubMed]
- Okino Y, Kiyosue H, Mori H, et al. Root of the Small-Bowel Mesentery: Correlative Anatomy and CT Features of Pathologic Conditions. Radiographics 2001;21:1475-90. [Crossref] [PubMed]
- Haak BW, Bodewitz ST, Kuijper CF, et al. Intestinal malrotation and volvulus in adult life. Int J Surg Case Rep 2014;5:259-61. [Crossref] [PubMed]
- Appaji AC, Kulkarni R, Kadaba JS. Nonrotation of Intestine: A Case Report. J Clin Diagn Res 2013;7:2575-6. [PubMed]
- von Flue M, Herzog U, Ackermann C, et al. Acute and chronic presentation of intestinal nonrotation in adults. Dis Colon Rectum 1994;37:192-8. [Crossref] [PubMed]
- Mohan P, Ramamoorthy M, Venkataraman J. Nonrotation of the intestine. CMAJ 2008;179:49-50. [Crossref] [PubMed]
- Pumberger W, Kargl S. Malposition of the intestine malposition malrotation volvulus "midgut volvulus". European Surgery 2012;44:237-47. [Crossref]
- Bozlar U, Ugurel MS, Ustunsoz B, et al. CT Angiographic Demonstration of a Mesenteric Vessel "Whirlpool" in Intestinal Malrotation and Midgut Volvulus: a Case Report. Korean J Radiol 2008;9:466-9. [Crossref] [PubMed]
- Peterson CM, Anderson JS, Hara AK, et al. Volvulus of the Gastrointestinal Tract: Appearances at Multimodality Imaging. Radiographics 2009;29:1281-93. [Crossref] [PubMed]
- Akinkuotu A, Samuel JC, Msiska N, et al. The Role of the Anatomy of the Sigmoid Colon in Developing Sigmoid Volvulus: A Case-Control Study. Clin Anat 2011;24:634-7. [Crossref] [PubMed]
- Michael SA, Rabi S. Morphology of Sigmoid Colon in South Indian Population: A Cadaveric Study. J Clin Diagn Res 2015;9:AC04-AC7. [PubMed]
- Cirocchi R, Farinella E, La Mura F, et al. The sigmoid volvulus: surgical timing and mortality for different clinical types. World J Emerg Surg 2010;5:1. [Crossref] [PubMed]
- Hasbahceci M, Basak F, Alimoglu O. Cecal Volvulus. Indian J Surg 2012;74:476-9. [Crossref] [PubMed]
- Lee SY, Bhaduri M. Cecal volvulus. CMAJ 2013;185:684. [Crossref] [PubMed]
- Berrocal T, Lamas M, Gutiérrez J, et al. Congenital Anomalies of the Small Intestine, Colon, and Rectum. Radiographics 1999;19:1219-36. [Crossref] [PubMed]
- Applegate KE, Anderson JM, Klatte EC. Intestinal Malrotation in Children: A Problem-solving Approach to the Upper Gastrointestinal Series. Radiographics 2006;26:1485-500. [Crossref] [PubMed]
- O'Connell PR, Madoff RD, Solomon M. Operative Surgery of the Colon, Rectum and Anus, Sixth Edition. CRC Press, 2015.
- Tackett JJ, Muise ED, Cowles RA. Malrotation: Current strategies navigating the radiologic diagnosis of a surgical emergency. World J Radiol 2014;6:730-6. [Crossref] [PubMed]
- Nehra D, Goldstein AM. Intestinal malrotation: varied clinical presentation from infancy through adulthood. Surgery 2011;149:386-93. [Crossref] [PubMed]
- Dilley AV, Pereira J, Shi EC, et al. The radiologist says malrotation: does the surgeon operate? Pediatr Surg Int 2000;16:45-9. [Crossref] [PubMed]
- Long FR, Kramer SS, Markowitz RI, et al. Radiographic patterns of intestinal malrotation in children. Radiographics 1996;16:547-56; discussion 556-60. [Crossref] [PubMed]
- McVay MR, Kokoska ER, Jackson RJ, et al. Jack Barney Award. The changing spectrum of intestinal malrotation: diagnosis and management. Am J Surg 2007;194:712-7; discussion 718-9. [Crossref] [PubMed]
- Vural V, Türkoğlu MA, Karatas G. Incidental midgut malrotation detected during second laparotomy: Case report and literature review. Int J Surg Case Rep 2015;7:134-6. [Crossref] [PubMed]
- Zissin R, Rathaus V, Oscadchy A, et al. Intestinal malrotation as an incidental finding on CT in adults. Abdom Imaging 1999;24:550-5. [Crossref] [PubMed]
- Al-Salem AH. Intestinal Malrotation. An Illustrated Guide to Pediatric Surgery. Cham: Springer International Publishing, 2014:173-80.
- Soop M, Spinelli A. What is at the Cutting Edge of IBD? Proceedings of the European Crohn's and Colitis Organisation 2018 Congress from a Surgical Perspective. Dis Colon Rectum 2018;61:879-82. [Crossref] [PubMed]
- Smida M, Miloudi N, Hefaiedh R, et al. Emergency surgery for Crohn's disease. Tunis Med 2016;94:210-5. [PubMed]
- Kim YS, Jung SA, Lee KM, et al. Impact of inflammatory bowel disease on daily life: an online survey by the Korean Association for the Study of Intestinal Diseases. Intest Res 2017;15:338-44. [Crossref] [PubMed]
- Coffey JC, O'Leary DP, Kiernan MG, et al. The mesentery in Crohn's disease: friend or foe? Curr Opin Gastroenterol 2016;32:267-73. [Crossref] [PubMed]
- Norouzinia M, Chaleshi V, Alizadeh AHM, et al. Biomarkers in inflammatory bowel diseases: insight into diagnosis, prognosis and treatment. Gastroenterol Hepatol Bed Bench 2017;10:155-67. [PubMed]
- Batra A, Stroh T, Siegmund B. Extraluminal factors contributing to inflammatory bowel disease. World J Gastroenterol 2011;17:572-7. [Crossref] [PubMed]
- Sheehan AL, Warren BF, Gear MW, et al. Fat-wrapping in Crohn's disease: pathological basis and relevance to surgical practice. Br J Surg 1992;79:955-8. [Crossref] [PubMed]
- Amitai MM, Ben-Horin S, Eliakim R, et al. Magnetic resonance enterography in Crohn's disease: A guide to common imaging manifestations for the IBD physician. J Crohns Colitis 2013;7:603-15. [Crossref] [PubMed]
- Coffey JC, Kiernan MG, Sahebally SM, et al. Inclusion of the mesentery in ileocolic resection for Crohn's disease is associated with reduced surgical recurrence. J Crohns Colitis 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Buskens CJ, de Groof EJ, Bemelman WA, et al. The role of the mesentery in Crohn's disease. Lancet Gastroenterol Hepatol 2017;2:245-6. [Crossref] [PubMed]
- Li Y, Zhu W, Gong J, et al. The role of the mesentery in Crohn's disease. Lancet Gastroenterol Hepatol 2017;2:244-5. [Crossref] [PubMed]
- Kirchhoff P, Clavien PA, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Saf Surg 2010;4:5. [Crossref] [PubMed]
- Kin C. Complex and Reoperative Colorectal Surgery: Setting Expectations and Learning from Experience. Clin Colon Rectal Surg 2016;29:75-9. [Crossref] [PubMed]
- Pérez-Guerra JA, Vázquez-Hernández M, Ramírez-Moreno R, et al. Abdominal re-operations: Prevalence in elective and emergency surgery. Cir Cir 2017;85:109-13. [PubMed]
- Coffey JC, Sehgal R, Walsh D. Mesenteric considerations in reoperative abdominal surgery. Mesenteric Principles of Gastrointestinal Surgery: Basic and Applied Science. CRC Press, 2017:333-43.
- Tabibian N, Swehli E, Boyd A, et al. Abdominal adhesions: A practical review of an often overlooked entity. Ann Med Surg (Lond) 2017;15:9-13. [Crossref] [PubMed]
- Gomel V, diZerega G, DeCherney AH. Peritoneal Surgery. New York: Springer, 2000.
- Mizrahi S, Reissman P, Polk HC. Reoperative Abdominal Surgery. JP Medical Limited, 2014.
- Feigel A, Sylla P. Role of Minimally Invasive Surgery in the Reoperative Abdomen or Pelvis. Clin Colon Rectal Surg 2016;29:168-80. [Crossref] [PubMed]
- Complex Kin C., Surgery Reoperative Colorectal. Clin Colon Rectal Surg 2016;29:73-4. [Crossref] [PubMed]
- Coffey JC, Sehgal R, Walsh D. Mesenteric considerations in ostomy formation and reversal. Mesenteric Principles of Gastrointestinal Surgery: Basic and Applied Science. CRC Press, 2017:323-33.
- Hendren S, Hammond K, Glasgow SC, et al. Clinical practice guidelines for ostomy surgery. Dis Colon Rectum 2015;58:375-87. [Crossref] [PubMed]
- Coffey JC, Sehgal R, Walsh D. Radiographic appearance of the mesentery and peritoneum. Mesenteric Principles of Gastrointestinal Surgery: Basic and Applied Science. CRC Press, 2017:109-19.
- Charnsangavej C, DuBrow RA, Varma DG, et al. CT of the mesocolon. Part 1. Anatomic considerations. Radiographics 1993;13:1035-45. [Crossref] [PubMed]
- Charnsangavej C, Dubrow RA, Varma DG, et al. CT of the mesocolon. Part 2. Pathologic considerations. Radiographics 1993;13:1309-22. [Crossref] [PubMed]
- Culligan K, Sehgal R, Mulligan D, et al. A detailed appraisal of mesocolic lymphangiology--an immunohistochemical and stereological analysis. J Anat 2014;225:463-72. [Crossref] [PubMed]
Cite this article as: Sehgal R, Connelly TM, Mohan HM, Byrnes GJ, Peirce C, Coffey JC. The importance of the mesentery in emergency general surgery: ignore the mesentery at your peril. Mesentery Peritoneum 2018;2:4.


