
Proof-of-concept assessment of metastatic sentinel node involvement by 18F-FDG positron emission tomography/computerized tomography and prediction of disease progression and survival in colorectal cancer patients with peritoneal carcinomatosis
Introduction
Peritoneal carcinomatosis (PC) has long been recognized as a poor prognostic indicator for colorectal cancer (CRC) patients, usually with an expectant survival of less than 1 year (1,2). However, there has been a recent paradigm change in approaches to management from palliative therapy to treatment with curative intent due to significant survival benefit derived from multimodal treatment strategies using combinations of cytoreductive surgery, hyperthermic intraoperative chemotherapy (HIPEC) and systemic chemotherapy (3-12). Data suggest that aggressive treatment of PC in carefully selected patients may improve both survival and quality of life (10,13).
Risk stratification of PC for CRC patients relies on a combination of intra- and post-operative indicators such as the peritoneal cancer index (PCI) (14) and completeness of cytoreduction score (CCS) (6). These are powerful indictors but they can only be assessed during or after the peritoneal surgical procedure (15). PCI is defined by summation of the one-dimensional size of the largest PC lesion in divided regions of the abdomen assigned either intra-operatively or by cross-sectional imaging (16-20). It does not take into consideration metabolic or other tumor characteristics. Thus, 18F-FDG positron emission tomography (PET)/CT has emerged as an improved hybrid imaging modality for assessment of PC (21-27).
A good understanding of physio-anatomical mechanism governing the lymphatic drainage and inter-compartmental fluid flow of the peritoneum is essential for evaluation of PC burden in CRC patients. In order to fully utilize the quantitation capability of PET and the functional ability of PET tracers, we aimed to determine the temporal pattern of the detection of positive portal, celiac or superior mesenteric nodes and to correlate this with the detection and volume of peritoneal carcinomatosis. We also aimed to assess the prognostic significance of portal, celiac and superior mesenteric nodal positivity.
Methods
Patients
All patients were followed by PET/CT in our center post resection of primary colonic malignancy. From 2012, 72 patients [32 men, 40 women; mean age: 65 (range 51–79) years] were suitable for inclusion as they developed evidence of either PCS or PC in the absence of evidence of anastomotic or local disease recurrence. The study was approved by our institutional ethics committee (IRB) and all patients gave written informed consent. All patients were followed with serial PET/CT studies for 3 years (scan interval 3–9 months) or till death. The order of the first detection of metastatic PCS lymph nodes, if present, was recorded with reference to that of PC. Based on the temporal relationship between detection of PC and PCS nodes on serial scans, these patients were categorized into 4 groups: occurrence of hypermetabolic PCS nodes before PC (Group I), along with PC (Group II), after PC (Group III), or PC alone without PCS nodes (Group IV) during follow-up.
PET/CT imaging
All patients fasted for at least 6 hours and blood glucose concentration (accepted level <8 mmol/L) was determined before injection of PET radiopharmaceuticals. A dose of 18F-FDG adjusted per patient’s weight was injected intravenously (333–518 MBq; 6.3 MBq/kg). Limited whole-body PET/CT (mCT, Siemens, Knoxville, TN, USA) was performed at 70~80 minutes after injection, from the skull base to the upper thigh.
Interpretation criteria and statistical analysis
PC was diagnosed on PET/CT based on visual identification of focal and/or diffuse hypermetabolic peritoneal lesions in the abdomen and pelvis. Focal PC was defined as discrete/confluent non-visceral foci of increased 18F-FDG metabolism within the abdominal and pelvic cavities, spatially confirmed by CT as lesions along the peritoneal reflections, mesentery or omentum, particularly in predilection sites prone to develop loculation or sediment deposition. These include subphrenic spaces, bilateral paracolic gutters, lesser sac, right hepatorenal space, ileocaecal recess and Pouch of Douglas. A serosal metastasis was diagnosed in the presence of nodular hypermetabolic activity contiguous with the serosal side of bowel wall and that on delayed scan. Subhepatic peri-capsular PC, when suspected, was confirmed by an additional breath-hold regional scan performed with time-of-flight technique to differentiate from liver metastasis. Hypermetabolic lymph nodes in the celiac, portal or SMA (PCS) lymphatic stations and within retroperitoneum were not considered as PC. Diffuse PC was first scanned for by inspecting the maximal intensity projection (MIP) images on PET to look for loss of visceral outlines, poor visualization of physiologic bowel activities or background vertebral segmentation. Features suggestive of diffuse PC included a rim of accentuated activity along the inner abdominal/pelvic walls on the orthogonal PET images, patchy ill-defined foci corresponding to cloudy opacities, engorged mesenteric vessels or fatty stranding on CT. Ascites alone was not considered as diffuse PC.
Confirmation of PC and PCS nodes was by (I) histopathological results after cytoreductive surgery (n=29); (II) serial PET/CT imaging in 3 months showing increased lesion size and/or tracer accumulation, supported by elevated serum CEA level (n=39); (III) additional CT findings of infiltration, thickening, or studding of the peritoneum, bowel wall, mesentery or omentum (n=14).
Quantitation of functional PC burden
Based on the above interpretation criteria, the total lesion glycolysis (TLG) of each confirmed PC lesion was then quantified by,
where and are the volume and SUV of ith voxel. is the number of voxels within the ROI, which is drawn automatically by the commercial software MIMContouring 4.2 to minimize operator-dependent bias. We defined PCTLG as the summation of TLG of all PC lesions, a parameter possessing both volumetric and metabolic quantitative information for assessment of PC burden. In cases where diffuse PC was suspected, quantitation was only performed on lesions that could be meaningfully defined and with successful ROI boundary delineation by MIMContouring.
One-way ANOVA was used to evaluate the significant difference of PCTLG among Groups I, II and III. An unpaired student t-test was used to compare the PCTLG of Groups I + II + III and IV.
Determination of the relationship between PCS nodal positivity and the presence and magnitude of PC
The correlation between the PCTLG and presence/absence of metastatic (hypermetabolic) PCS nodes on serial PET/CT was analyzed using the Pearson’s correlation. A PCTLG threshold was identified that correlated with the detection of PCS nodal positivity in the setting of peritoneal carcinomatosis, using receiver operating characteristic (ROC) curve analysis.
Parameters for predicting 3-year overall survival (OS) in CRC patients with PC
Multivariate Cox regression analysis (SPSS 14.0) was performed on several parameters to evaluate prognostic significance in predicting 3-year OS in CRC patients with PC: serum CEA concentration, presence/absence of liver metastasis, presence/absence of metastatic PCS lymph nodes, presence/absence of metastatic left supraclavicular (LSCF) lymph nodes, and PCTLG.
Results
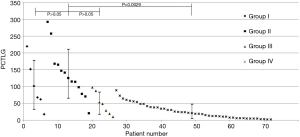
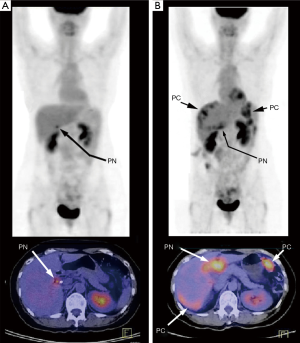
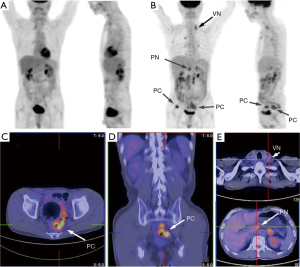
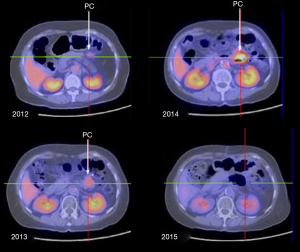
During PET/CT follow-up of CRC patients post primary resection, 72 patients developed peritoneal disease recurrence. The primary tumor characteristics are listed in Table 1. Of the 72 patients recruited into this longitudinal study, 46 (63.9%) were found to have PC only (Group IV), while 26 (36.1%) had both PC and positive PCS nodes: 6 in Group I, 13 in Group II and 7 in Group III. Figure 1 plots the PCTLG for all groups. The mean PCTLG for Groups I, II, III, and IV were: 104±72, 138±73, 50±33 and 26±21, respectively. Group IV patients (PCS node negative) had the lowest PCTLG score compared with those of the other three groups with positive PCS nodes (mean PCTLG =106±73 vs. 26±21, P<0.05); however, no statistically significant difference was found in PCTLG scores between Groups I, II and III patients (one-way ANOVA, P>0.05). On ROC analysis, PCTLG ≥27 was associated with positive PCS nodes with a sensitivity of 84.6% and specificity of 71.7% (AUC =0.776, P<0.05). Figure 2 relates to a Group I patient with a hypermetabolic PCS node on first PET/CT but without evidence of PC. Six months later despite treatment, serial PET/CT showed progression of the PCS node and peritoneal carcinomatosis (PCTLG =68). Figure 3 relates to a Group II patient with positive PCS and Virchow’s nodes and multifocal PC at initial presentation (PCTLG =142). Figure 4 relates to a Group III patient after resection of a locally-advanced colonic carcinoma. Intra-operatively, a number of PC nodules were confirmed although PET/CT on day 3 after surgery was negative (Figure 4A). PC was detected on follow-up PET/CT (Figure 4B,C,D,E) while portal and Virchow’s nodes were also detected. Figure 5 presents a Group IV patient with negative PCS nodes at the time when peritoneal carcinomatosis was detected (in 2012) (PCTLG =5). From 2012 to 2014, serial PET/CT scans showed low-volume PC progression. After cytoreductive surgery and chemotherapy, no tumor recurrence was found on subsequent follow-up.
Full table

In this study, 33/72 patients (45.8%) survived longer than 3 years. Univariate analysis identified 3 parameters: PCTLG, positive PCS nodes and positive LSCF nodes (all P<0.05) as significant predictors of 3-year OS, while CEA and liver metastasis were not (both P>0.05). Multivariate analysis of these 3 predictors showed that only positive PCS nodes and PCTLG were independent predictors of survival.
Patients with positive PCS nodes had significantly shorter survival and lower 3-year OS rate than those without (median survival =16 months vs. >3 years, P<0.05; OS rate =7.7% vs. 67.4%, Figure 6A). Similarly, PCTLG score ≥27 predicted poor 3-year survival with a sensitivity of 76.9% and specificity of 84.8% on ROC analysis (AUC =0.834, P<0.05). Patients with PCTLG ≥27 had a median survival of 18 months and 14.3% 3-year OS rate, as compared to a median survival >3 years and 75.7% 3-year OS rate for those with PCTLG <27 (P<0.05; Figure 6B).
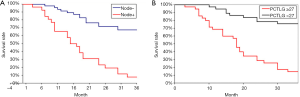
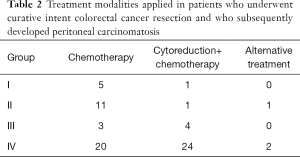
Table 2 summarizes modes of treatment received by the 4 groups of patients based on PET/CT parameters and findings. Of the 26 PC patients with positive PCS nodes (Groups I + II + III), 20/26 were treated by medical therapy (chemotherapy 19/20, alternative therapy 1/20), and 6/26 by cytoreductive surgery. There was no significant difference in survival between the surgical group and a selected medical group (6 patients) with matched PCTLG scores (PCTLG mean =38.6±9.4 and median survival =24 months, vs. 37.8±12.2 and 22 months, respectively, P>0.05). On the contrary, of the 46 Group IV patients with negative PCS nodes, 22/33 patients with PCTLG <27 were ultimately treated by combined cytoreductive surgery and chemotherapy, and they had a significantly better 3-year OS rate (19/22=86.4%). For the 13 Group IV patients with PCTLG ≥27, their OS was not prolonged whether they had chemotherapy alone (11/13, median survival 19 months) or with additional cytoreductive surgery (2/13, average survival 18 months).
Full table
Discussion
Although the combined treatment of cytoreductive surgery and HIPEC have been shown to be efficacious in patients who develop peritoneal carcinomatosis following curative surgery for colorectal cancer (3,5,28), the selection of patients who may benefit from this strategy remains challenging (10). Predictive threshold values and classification scores related to PCI and CCS vary considerably between studies (15,29-31).
We proposed that current prognostic indicators may be improved on with incorporation of data regarding metabolic take-up, 3 dimensional size, and lymphatic drainage to portal, celiac or superior mesenteric nodes. We found that the PCTLG, and the presence of positive portal, celiac or superior mesenteric nodes, independantly predicted survival and hence affected prognosis in this patients with peritoneal carcinomatosis following curative resection for colorectal cancer. Matsuda et al. (32) suggested that additional factors such as lymph node ratio, and the extend of carcinomatosis were also poor prognosticators.
This study revealed that the percentage of patients with a high PCTLG score (≥27) was significantly greater in patients with PCS nodal metastasis than those without (84.6% vs. 28.3%, respectively). However, from the longitudinal data analysis of the sequential PET/CT for patients having positive PCS nodal metastasis, there was no statistically significant difference in PCTLG among individual groups (Groups I, II and III). In other words, if there is a metastatic PCS node detected on PET/CT in post-operative CRC patients, the chance of having co-existing PC is high, regardless of whether hypermetabolic peritoneal tumors can be concomitantly visualized on the PET images performed at that time point. Given peritoneal seedlings can be small, or diffusely distributed along regions of the reflection, the possibility of false negative PET/CT is relatively high. Based on experiments using animal models, Parungo et al. suggested that PCS nodes represent a “sentinel” station of lymphatic drainage from numerous regions of the peritoneum (33). Sentinel nodes are the first tier of nodes to which an organ drains lymph. Hence, they are postulated to be the first nodes which tumor cells reach, if they are metastasising along lymphatic channels. Mesenteric nodes provide a common route of drainage and lymphatic metastasis, once the visceral peritoneal lymphatic network has been breached. Given this, the presence of a hyper-metabolic PCS node has major implications and points to the development of peritoneal disease. This is reflected in the findings of the present study which note that on longitudinal follow-up of patients with positive PCS nodes, peritoneal carcinomatosis is usually detected, even though it may have been absent on earlier scans. Once PC becomes visible on PET/CT, these patients (Groups I, II and III) are usually found to have higher PCTLG score (PC burden) and a faster course of PC progression. On the contrary, the patients with negative PCS node (Group IV) usually have lower PCTLG score and a more indolent course of PC development. Of the 46 patients in this group, 31 were alive beyond 3 years of follow-up.
This study also identified a relationship between the presence of positive PCS node, and a positive Virchow’s node. This is concordant with the conventional belief that lymphatic drainage to Virchow’s node from portal nodes occurs via the thoracic duct (33). The presence of a posivitive Virchow’s node points to breakdown of portal lymphatic defense systems, rather than the previously postulated concept of a “skipped” nodal metastasis. The presence of a positive Virchow’s node and/or sister Mary Joseph’s umbilical nodule are accepted as amongst the worst prognostic indicators in patients with peritoneal carcinomatosis (34).
As OS is known to be affected by a number of factors including variations in therapeutic regimes, durations and presence of concomitant diseases unrelated to PC or complications from treatment, it is difficult to isolate any of these factors to assess individual contributions to prognosis. In this study, patients might not be treated by cytoreduction if pretreatment scan showed high tumor burden (PCTLG ≥27 or positive PCS nodes). Therefore, only 2 patients with a PCTLG greater than or equal to 27, and six patients with positive PCS nodes were treated by cytoreductive surgery. Our study suggests that there is a prognostic advantage (3-year OS rate of 86.4%) for patients with negative PCS nodes and PCTLG <27 and who undergo combined chemotherapy and cytoreductive surgery. A larger cohort study with better design of treatment criteria is needed to support our initial observation.
Knowledge of the pathophysiology and mechanisms governing lymphatic drainage of the peritoneum is important if one is to understand patterns of progression of peritoneal carcinomatosis. The detection of PCS nodal positivity on surveillance PET/CT following curative resection of a colorectal cancer may have important predictive and prognostic implications that will help in stratification of patients and tailoring of their treatment.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by our institutional ethics committee (IRB) and all patients gave written informed consent.
References
- Chu DZ, Lang NP, Thompson C, et al. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer 1989;63:364-7. [Crossref] [PubMed]
- Jayne DG, Fook S, Loi C, et al. Peritoneal carcinomatosis from colorectal cancer. Br J Surg 2002;89:1545-50. [Crossref] [PubMed]
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737-43. [Crossref] [PubMed]
- Ihemelandu CU, Shen P, Stewart JH, et al. Management of peritoneal carcinomatosis from colorectal cancer. Semin Oncol 2011;38:568-75. [Crossref] [PubMed]
- Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 2004;22:3284-92. [Crossref] [PubMed]
- Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg 1995;221:124-32. [Crossref] [PubMed]
- Franko J, Ibrahim Z, Gusani NJ, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer 2010;116:3756-62. [Crossref] [PubMed]
- Glockzin G, Ghali N, Lang SA, et al. Results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal cancer. J Surg Oncol 2009;100:306-10. [Crossref] [PubMed]
- Cotte E, Passot G, Mohamed F, et al. Management of peritoneal carcinomatosis from colorectal cancer: current state of practice. Cancer J 2009;15:243-8. [Crossref] [PubMed]
- O'Dwyer S, Verwaal VJ, Sugarbaker PH. Evolution of Treatments for Peritoneal Metastases From Colorectal Cancer. J Clin Oncol 2015;33:2122-3. [Crossref] [PubMed]
- Garcia JR, Villasboas-Rosciolesi D, Soler M, et al. Peritoneal Cancer Index by (18)F-FDG PET/TC pre and post-hyperthermic intraperitoneal chemotherapy. Report of a case. Rev Esp Med Nucl Imagen Mol 2016;35:329-31. [Crossref] [PubMed]
- Cistaro A, Cucinotta M, Cassalia L, et al. (18)F-FDG PET/CT, cytoreductive surgery and intraperitoneal chemohyperthermia for the therapeutic management in peritoneal carcinomatosis: A pilot study. Rev Esp Med Nucl Imagen Mol 2016;35:232-7. [Crossref] [PubMed]
- Sugarbaker PH. Patient selection and treatment of peritoneal carcinomatosis from colorectal and appendiceal cancer. World J Surg 1995;19:235-40. [Crossref] [PubMed]
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996;82:359-74. [Crossref] [PubMed]
- Goldstein P, Gomes de Silva R, Cabanas J, et al. Management of peritoneal carcinomatosis from colon cancer, gastric cancer and appendix malignancy. Cancer Ther 2005;3:299-320.
- Ricke J, Sehouli J, Hach C, et al. Prospective evaluation of contrast-enhanced MRI in the depiction of peritoneal spread in primary or recurrent ovarian cancer. Eur Radiol 2003;13:943-9. [PubMed]
- Marin D, Catalano C, Baski M, et al. 64-Section multi-detector row CT in the preoperative diagnosis of peritoneal carcinomatosis: correlation with histopathological findings. Abdom Imaging 2010;35:694-700. [Crossref] [PubMed]
- Esquivel J, Chua TC, Stojadinovic A, et al. Accuracy and clinical relevance of computed tomography scan interpretation of peritoneal cancer index in colorectal cancer peritoneal carcinomatosis: a multi-institutional study. J Surg Oncol 2010;102:565-70. [Crossref] [PubMed]
- Koh JL, Yan TD, Glenn D, et al. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol 2009;16:327-33. [Crossref] [PubMed]
- Esquivel J, Chua TC. CT versus intraoperative peritoneal cancer index in colorectal cancer peritoneal carcinomatosis: importance of the difference between statistical significance and clinical relevance. Ann Surg Oncol 2009;16:2662-3; author reply 264. [Crossref] [PubMed]
- Suzuki A, Kawano T, Takahashi N, et al. Value of 18F-FDG PET in the detection of peritoneal carcinomatosis. Eur J Nucl Med Mol Imaging 2004;31:1413-20. [Crossref] [PubMed]
- Tanaka T, Kawai Y, Kanai M, et al. Usefulness of FDG-positron emission tomography in diagnosing peritoneal recurrence of colorectal cancer. Am J Surg 2002;184:433-6. [Crossref] [PubMed]
- Bamba Y, Itabashi M, Kameoka S. Clinical Use of PET/CT in Peritoneal Carcinomatosis from Colorectal Cancer. Hepatogastroenterology 2012;59:1408-11. [PubMed]
- Berthelot C, Morel O, Girault S, et al. Use of FDG-PET/CT for peritoneal carcinomatosis before hyperthermic intraperitoneal chemotherapy. Nucl Med Commun 2011;32:23-9. [Crossref] [PubMed]
- Pfannenberg C, Konigsrainer I, Aschoff P, et al. (18)F-FDG-PET/CT to select patients with peritoneal carcinomatosis for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2009;16:1295-303. [Crossref] [PubMed]
- Blodgett TM, Meltzer CC, Townsend DW. PET/CT: form and function. Radiology 2007;242:360-85. [Crossref] [PubMed]
- Turlakow A, Yeung HW, Salmon AS, et al. Peritoneal carcinomatosis: role of (18)F-FDG PET. J Nucl Med 2003;44:1407-12. [PubMed]
- Jacquet P, Stephens AD, Averbach AM, et al. Analysis of morbidity and mortality in 60 patients with peritoneal carcinomatosis treated by cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy. Cancer 1996;77:2622-9. [Crossref] [PubMed]
- Pestieau SR, Sugarbaker PH. Treatment of primary colon cancer with peritoneal carcinomatosis: comparison of concomitant vs. delayed management. Dis Colon Rectum 2000;43:1341-6; discussion 7-8. [Crossref] [PubMed]
- Elias D, Blot F, El Otmany A, et al. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer 2001;92:71-6. [Crossref] [PubMed]
- Elias D, Bonnay M, Puizillou JM, et al. Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: pharmacokinetics and tissue distribution. Ann Oncol 2002;13:267-72. [Crossref] [PubMed]
- Matsuda K, Hotta T, Takifuji K, et al. Lymph nodes ratio is associated with the survival of colorectal cancer patients with peritoneal carcinomatosis. Am Surg 2011;77:602-7. [PubMed]
- Parungo CP, Soybel DI, Colson YL, et al. Lymphatic drainage of the peritoneal space: a pattern dependent on bowel lymphatics. Ann Surg Oncol 2007;14:286-98. [Crossref] [PubMed]
- Chua TC, Yan TD, Ng KM, et al. Significance of lymph node metastasis in patients with colorectal cancer peritoneal carcinomatosis. World J Surg 2009;33:1488-94. [Crossref] [PubMed]
Cite this article as: Ho CL, Chen S, Leung YL, Cheng KC, Wong KN, Cheung SK, Wong YH. Proof-of-concept assessment of metastatic sentinel node involvement by 18F-FDG positron emission tomography/computerized tomography and prediction of disease progression and survival in colorectal cancer patients with peritoneal carcinomatosis. Mesentery Peritoneum 2019;3:1.


