Primary mesorectal carcinoid tumour, a case report and review of literature
Introduction
Carcinoid tumours of unknown origin (CUO) account for 10% for all neuroendocrine tumours (NETs) (1). Lack of a primary source poses difficulties with regard to optimum treatment strategy for these tumours and prediction of future metastatic recurrence. Utilisation of 68-gallium Dotatate PET-CT offers increased sensitivity and characterization of CUO, compared to traditional octreotide scans (2). Development of this may allow investigation into the mesentery as a potential primary site.
The mesentery is now known to have the characteristics that are required of an organ (3). Therefore carcinoid deposits that develop in the mesentery may be defined as primary mesenteric carcinoids and not CUO as previously described. Documentation of primary mesenteric carcinoid tumours are beginning to increase. Barnardo et al. (4) published one of the first case reports, detailing intestinal obstruction secondary to a primary carcinoid tumor of the mesentery. At this time primary mesenteric carcinoid tumours were hypothetical but further evidence from Karahan et al. (5), Yamanuha et al. (6) and Park et al. (7) provide support for the idea that the mesentery may be a site for primary carcinoid development. Most recently Shogbesan et al. (8) described a 64 years old male with carcinoid syndrome arising from primary mesenteric carcinoid tumour.
The European Neuroendocrine Tumour Society (ENETS) strives to generate gold standard guidelines for the treatment and management of all carcinoid tumours (9). However to date there is no reference to primary mesenteric or mesorectal carcinoid tumours. In the absence of correct classification and guidelines on mesenteric carcinoids it is difficult to know how best to survey and manage such patients following initial curative resection. Herein we describe the case of primary mesorectal carcinoid tumour with interval nodal recurrence and the treatment and management plan undertaken.
Case presentation
A 55 years old female was referred to the colorectal outpatient clinic. She presented with symptoms of increasingly severe anal discomfort and progressive obstructive defecation over the previous four months with an associated seven pounds of unintentional weight loss. There were no symptoms of PR bleeding, abdominal pain, diarrhoea or tenesmus described. She had nil significant past medical and surgical histories. Her father was diagnosed with colorectal carcinoma aged 80. Abdominal examination was unremarkable with no palpable systemic lymphadenopathy. Digital rectal examination identified a posterior rectal mass with normal smooth mucosa overlying the mass. Prompt haematological and endoscopic investigations were normal.
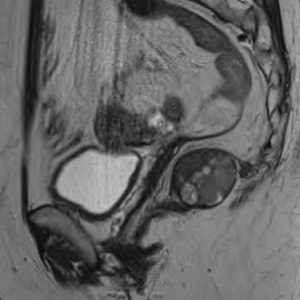
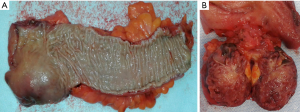
Computed tomography (CT) thorax abdomen pelvis (TAP) revealed a 4.2 cm (AP) × 3.8 cm (transverse) predominantly solid appearing lesion in the right ischiorectal fossa, a clear plane was not visible between the mass and the upper 3rd of rectum and with the mass infiltrating the surrounding fat of the mesorectum. There was no associated local lymphadenopathy evident or distant metastasis. Subsequent MRI pelvis further defined this as a 3.5 cm well defined, encapsulated, lobulated mass confined to the right ischioanal space, but closely applied to the undersurface of the levator plate (Figure 1). A CT-guided transanal biopsy confirmed a well differentiated neuroendocrine tumour, grade 1. Positive tumour markers included: CD56, Synaptophysin & Chromogranin and a low proliferation index (1–2%) was reported. Serum chromogranin A levels were also elevated at 122 ng/mL (27–94 ng/mL). Based on the above findings a diagnosis of carcinoid tumour was made. The tumour was located within the mesorectum but distinct from the rectum itself therefore the nomenclature of a primary mesorectal carcinoid was assigned. An octreotide scan was performed and was normal with some scattered foci in the pelvis. Thus locoregional disease was outruled and surgical resection planned as definitive treatment. Due to the close proximity of the tumour to the rectum a laparoscopic ultra-low anterior resection with ileostomy formation was performed. Histopathology results of the resected specimen (Figure 2), reported a well differentiated neuroendocrine tumour, grade 1 with lymphovascular & perineural invasion. Mitotic index was <1/10 high power fields & Ki-67 <2%. Five lymph nodes were negative for malignant disease.
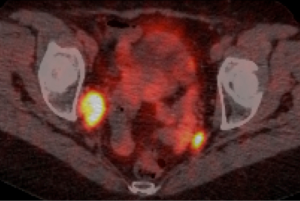
Post-operative recovery was uncomplicated. Following discussion at the gastrointestinal multidisciplinary team meeting (GI MDM) a plan for standard colorectal cancer surveillance was agreed upon, comprising of annual CT TAP and colonoscopy. First CT TAP and colonoscopy were normal. However the second surveillance CT TAP highlighted new regional lymphadenopathy with a 22 mm × 15 mm enlarged lymph node in right external iliac chain adjacent to pelvic side wall and obturator internus. Nuclear imaging SPECT Octreoscan further concluded the right obturator internus enlarged lymph node to be octreotide avid, with a small subnormal focus uptake in the left hemi-pelvis. The case was referred for discussion at a national dedicated NET MDM and a recommendation for a gallium PET scan made. Whole body Gallium PET CT reported an intensely avid 17 mm right obturator lymph node and left internal inguinal and presacral nodes (Figure 3). Further MDM recommendations was for lymphadenectomy of the involved nodes prior to consideration of systemic therapy. Laparoscopic right obturator lymphadenectomy was performed and histopathology results confirmed metastatic well differentiated neuroendocrine tumour, 3 cm maximum dimension in one of seven lymph nodes resected. Immunohistochemistry further illustrated positive staining of the neoplastic cells for neuroendocrine markers: CD 56, synaptophysin and chromogranin. Post-operative recovery was uncomplicated and as such systemic therapy is not necessary. Annual CT AP surveillance will continue.
Discussion
Primary mesorectal carcinoid tumours have not yet been described in the literature. However since the classification of the mesentery as an organ in 2017, carcinoid tumours occurring in the mesentery should be considered as primary mesenteric carcinoids rather than the previous term of CUO when a solid organ is not involved. Carcinoid tumours are a specific type of neuroendocrine tumour (NET) originating from enterochromaffin cells, which are a subset of Amine Precursor Uptake Cell (APUD) series (derivatives of neural crest cells) (10). Neural crest cells migrate along the dorsal mesogastrium in the early embryonic period to reach their target locations in the primordial respiratory and gastrointestinal tracts (11). However should molecular signalling pathways that direct this process be disrupted it is possible that nests of APUD cells become dispersed in the dorsal mesogastrium, which subsequently could result in primary mesenteric carcinoid tumours. However this theory has yet to be investigated.
Primary mesorectal carcinoid tumours effectively illustrate the anatomy of the mesorectum. Superiorly, the mesorectum is formed by the union of the mesosigmoid at the pelvic brim (3). This continues inferiorly ending at the pelvic floor, where it has a caudal connection with Waldeyers fascia, now thought to be contiguous with Toldt’s fascia (12). This caudal connection is approximately 3–5 cm from the anorectal junction and thus is the most inferior extension of the mesorectum. The anatomical boundaries of this case’s tumour reflect these parameters, abutting the levator plate. As such future radiographic identification of lesions within these anatomical limitations should prompt the consideration of a mesorectal carcinoid.
With the evolution of 68-Gallium Dotatate PET CT imaging detection and characterization of primary mesenteric carcinoid and morphology is possible. This is due to the improved image resolution and ability to detect smaller lesions, with a 93% sensitivity compared to the Octreoscan, which has a sensitivity of 75% (2) in identifying carcinoid tumours and deposits. Previously described as CUO, enhanced understanding of the mesentery and improved imagining modalities can now support the mesentery as a primary site of carcinoid development and growth as in this case. In 2015, Kamath et al. (13) also highlighted a case of two primary mesenteric carcinoid tumours. Initially when identified the tumours were thought to be CUO, however utilisation of Dotatate positron emission tomography (PET) delineated a second tumour in the mesenteric root with no solid organ involvement. This confirmed the primary source to be the mesentery. Both Kamath et al. (13) and our case reports are comparable and suggest that mesenteric carcinoid tumours should be differentiated in patients with non-specific, progressive gastrointestinal (GI) symptoms and normal endoscopic investigations.
Currently European Neuroendocrine Tumour Society (ENETS) guidelines do not reference primary carcinoid tumours or their specific management. While it is likely the primary treatment of surgical excision will remain unchanged, the more discriminating factors of number of lymph node resected and management of recurrence needs to be formally documented. In the case of primary mesorectal carcinoid, a total mesorectal excision (TME) with the inclusion of at least twelve lymph nodes would at least theoretically offer the best option for a successful oncological resection when abiding by colorectal cancer management standards (14). However as is clear from this case, primary mesenteric tumours particularly of the hindgut present a challenge with regard to the pattern of metastasis.
The lymphatic contiguity between the mesentery and the intestine allows for the spread of disease (15). This intimate relationship can also allow for systematic calculation as to the target area for metastasis. Primary mesenteric carcinoid tumours that develop in the fore- or midgut have been shown to present with carcinoid syndrome or liver metastasis (16). This can be explained by the embryological development of the mesentery (12). Fore- and midgut organs, including the liver develop in the ventral mesogastrium, while the hindgut develops in the dorsal mesogastrium (11). The root of the mesentery is just left of the midline behind the second part of the duodenum, at the origin of the superior mesenteric artery (15). The lymphovascular supply of the GI tract passes through the mesenteric root and drains to the liver via the portal system. Hence it can be hypothesized that direct metastatic spread from the fore- and midgut mesentery to the liver is so frequent due to their embryological development and consequential direct hepatic drainage via the mesenteric root.
However, mesorectal lymphatic drainage may not follow such a definitive pathway as they have not yet reached the mesenteric root. At the end of the embryonic period, retroperitoneal lymph sac at the mesenteric root on the posterior abdominal wall is described, draining the primordial gut, with the cisterna chyli located dorsally (11). Subsequently these develop into groups of lymph nodes. Therefore before the hindgut drains into the mesenteric root, there a number of lymph node groups into which a primary mesorectal carcinoid tumour could potentially metastasize. This is of prognostic importance and is a current topic of interest in rectal cancer. Dev et al. (17) first suggested the concept of a ‘vulnerable field’ in lower rectal cancers, which identifies that lateral pelvic lymph node metastasis most commonly, can occur in the obturator space. It is postulated this involves a process enabled by extension of the rectal lymphatics via the lateral ligament passing through the obturator space towards the internal iliac artery (17). Fujita and Yamaguchi (18) and Beppu et al. (19) detail right sided obturator node metastasis after treatment for rectal cancer. These observations are comparable to those of the present case report. Lateral pelvic lymph node adenectomy would be the primary mode of treatment for this. However when multiple areas of metastasis exist, the diameter of the affected nodes is the most efficient pre-operative method to determine the effectiveness of lateral pelvic lymph node dissection (20). Furthermore this tendency toward right-sided obturator nodal metastasis may reflect the asymmetric process of lymphangiogenesis (21).
Conclusions
Primary mesenteric carcinoid tumours and primary mesorectal carcinoid tumours pose a number of challenges as highlighted by this case. Correctly identifying and naming the primary site, optimum operative approach and patient counselling regarding follow-up and surveillance require MDM discussion and consensus. With ever expanding knowledge of gastrointestinal anatomy based on mesenteric principles, previously unexplained tumour aetiology such as CUO need re-evaluation and the mesentery may be considered a primary source in such cases.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Scarsbrook AF, Ganeshan A, Statham J, et al. Anatomic and functional imaging of metastatic carcinoid tumors. Radiographics 2007;27:455-77. [Crossref] [PubMed]
- Geijer H, Breimer LH. Somatostatin receptor PET/CT in neuroendocrine tumours: update on systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 2013;40:1770-80. [Crossref] [PubMed]
- Coffey JC, O'Leary DP. The mesentery: structure, function, and role in disease. Lancet Gastroenterol Hepatol 2016;1:238-47. [Crossref] [PubMed]
- Barnardo DE, Stavrou M, Bourne R, et al. Primary carcinoid tumor of the mesentery. Hum Pathol 1984;15:796-8. [Crossref] [PubMed]
- Karahan OI, Kahriman G, Yikilmaz A, et al. Gastrointestinal carcinoid tumors in rare locations: imaging findings. Clin Imaging 2006;30:278-82. [Crossref] [PubMed]
- Yamanuha J, Ballinger R, Coon D, et al. Carcinoid tumor presenting as a primary mesenteric mass: a case report and review of the literature. Hawaii Med J 2009;68:137-9. [PubMed]
- Park IS, Kye BH, Kim HS, et al. Primary mesenteric carcinoid tumor. J Korean Surg Soc 2013;84:114-7. [Crossref] [PubMed]
- Shogbesan O, Abdulkareem A, Pappachen B, et al. Primary Mesenteric Carcinoid Tumor Presenting with Carcinoid Syndrome. Case Rep Gastroenterol 2018;12:396-401. [Crossref] [PubMed]
- Society E-ENT. 2019. Available online: https://www.enets.org/aims_misson.html. Accessed 13/06/19 2019.
- Pearse AG, Takor TT. Neuroendocrine embryology and the APUD concept. Clin Endocrinol (Oxf) 1976;5 Suppl:229S-44S. [Crossref] [PubMed]
- Sadler TW. Langman's medical embryology. Lippincott Williams & Wilkins; 2011.
- Byrnes KG, McDermott K, Coffey JC. Development of mesenteric tissues. Semin Cell Dev Biol 2019;92:55-62. [Crossref] [PubMed]
- Kamath SM, Pingali S, Girish G, et al. Primary synchronous mesenteric neuroendocrine tumors: Report of a rare case with review of literature. J Cancer Res Ther 2015;11:662. [Crossref] [PubMed]
- Engstrom PF, Arnoletti JP, Benson AB, et al. Colon cancer. J Natl Compr Canc Netw 2009;7:778-831. [Crossref] [PubMed]
- Coffey JC, Culligan K, Walsh LG, et al. An appraisal of the computed axial tomographic appearance of the human mesentery based on mesenteric contiguity from the duodenojejunal flexure to the mesorectal level. Eur Radiol 2016;26:714-21. [Crossref] [PubMed]
- Liang PS, Shaffer K. Metastatic Gastrointestinal Carcinoid Tumor with Unknown Primary Site. Radiol Case Rep 2015;2:90. [Crossref] [PubMed]
- Dev K, Jaglan N, Gurawalia J, et al. Lateral pelvic lymph node dissection in rectal cancer. Optimal treatment? Colorec Cancer 2015;1:1.
- Fujita S, Yamaguchi T. A Case of Lateral Pelvic Lymph Node Recurrence of Rectal Carcinoid. Jpn J Clin Oncol 2008;38:390. [Crossref] [PubMed]
- Beppu N, Niki M, Kimura F, et al. A case of rectal carcinoid, 7 mm in diameter, with skip metastasis to the lateral lymph node. Mol Clin Oncol 2016;4:549-52. [Crossref] [PubMed]
- Ueno H, Mochizuki H, Hashiguchi Y, et al. Potential prognostic benefit of lateral pelvic node dissection for rectal cancer located below the peritoneal reflection. Ann Surg 2007;245:80-7. [Crossref] [PubMed]
- Eberl G, Lochner M. The development of intestinal lymphoid tissues at the interface of self and microbiota. Mucosal Immunol 2009;2:478-85. [Crossref] [PubMed]
Cite this article as: Fullard A, Fleming CA, Condon E. Primary mesorectal carcinoid tumour, a case report and review of literature. Mesentery Peritoneum 2020;4:2.