
Post-surgical stratification of recurrence predictors for Crohn disease patients
Introduction
Crohn disease (CD) represents a chronic, idiopathic inflammatory disease which can alter any segment of the digestive tract, but usually involves the terminal ileum. The inflammatory process starts with mucosal damage and progresses to granulomatous transmural inflammation which presents an alternation between affected and healthy portions creating a macroscopic appearance characteristically known as “paving stone.”
The clinical progression of the disease is characterized by spontaneous flare phases followed by clinical remission. Approximately 75% of patients are exposed to flares while 10% will suffer from chronic complications and the rest will be asymptomatic for long periods (1,2).
At present, there is a wide range of treatments which can prevent flares some with greater efficacy than others. These include 5-ASA agents which are effective in high doses and come with a range of side effects which vary from nausea to headache and fatigue (3). Immunomodulators have been effective in patients who cannot be taken off steroids without a flare recurring. Long-term treatment is recommended (4). Biologic therapy such as infliximab or adalimumab has given the best results in treating moderate to severe CD and can be used in the long term to prevent flares (5).
Between 70% and 90% of patients diagnosed with CD will require surgery throughout their lifetime, while 39% will require reintervention (6).
Perioperative mortality of patients undergoing surgery ranges from 0% to 8.4%, and most of the deaths are secondary to sepsis with an intra-abdominal origin (7,8).
Different risk factors are associated with the first surgery, such as tobacco consumption, disease location, stenotic phenotype, and early use of high-dose immunomodulators or glucocorticoids (9).
The decision to operate a CD patient is not easy and should always be discussed in a multidisciplinary team that must include a gastroenterologist specialized in the treatment of inflammatory bowel disease. Mortality in emergency surgery is 2–3 times higher than surgery performed in elective situations (10).
Most patients with CD (90%) will require at least one life-saving surgery despite the available medical therapeutic arsenal, while 5% to 18% of these patients will suffer from short bowel syndrome and will require chronic parenteral nutrition which causes a significant decrease in quality of life (11,12). The risk of malabsorption increases significantly when the length of the small intestine is under 150 cm and is almost guaranteed when the small intestine reaches less than 100 cm (13).
Age, gender, the location of the disease, duration, and presence of granulomas, all have a different impact on postoperative recurrence; therefore, a risk factor other than smoking that could affect recurrence has not yet been established (14,15).
CD can be assisted by surgery but cannot be cured by surgery. The main goal of surgical interventions is to overcome the acute episode, to increase the quality of life for the patient and preserve the length of the digestive tract as much as possible, as the probability of postoperative recurrence 10 years after the first intervention is 25% to 45% (16).
Methods
Population
The study was structured as multicentric, and the group comprised 62 patients from five University Hospitals in Bucharest - Emergency Clinical Hospital, Prof. Dr. Agrippa Ionescu, Elias Emergency Clinical Hospital, St. Mary Clinical Hospital, Colentina Clinical Hospital, Fundeni Clinical Institute. Retrospective data collection began on 01.01.2008 to 08.08.2018. The mean follow-up period was 36 months.
Inclusion criteria
Positive diagnosis of CD and at least one surgical intervention due to CD complications, age 18 years.
Study material
Clinical observation data and surgical protocols, histopathological and imaging sources—laparoscopic video recordings, upper or lower digestive endoscopy, radiological investigations: computed tomography, entero-tomography, contrast radiographs, Pansdorf samples.
Variables
The following variables were used to create the database: age, gender, background (urban/rural), smoker status, diagnosis of CD, surgical indication, surgical technique, preoperative and postoperative medical therapy, post-operative evolution and complications, the context of surgery (elective/emergency).
Statistical analysis
SPSS version 23.0 and Microsoft Excel 2010 were used for statistical analysis. For the descriptive statistics, the mean and the standard deviation were calculated, namely the medians and quarts for the quantitative variables and the frequent and percentage qualitative variables. Quantitative data were tested to verify the normality and homogeneity of variants with the Levene test. For category data, exact Fisher tests and Pearson Chi-Square were used. For the investigation of the risk factors that differentiated the groups, the survival analysis (Log-Rank and Breslow tests) was also used, with the patient’s surgical recurrence, calculating the percentages of survivors (patients without recurrence). For multivariate analysis, binary logistic regression has been used.
The threshold for statistical significance was set at 0.05.
Results
We identified some 62 patients of which 27 were females (43.5%) with a median age of 40 years. The reintervention rate for surgical recurrence was 33%. From the study group, the majority of patients were smokers (72.6%). A number of n=25 (40.3%) patients had preoperative treatment which consisted of immunosuppression n=14 (22.6%), corticotherapy n=9 (14.5%), biologics n=6 (9.7%). Postoperative treatment consisted of immunosuppression n=14 (22.6%), corticotherapy n=28 (45.2%), biologics n=28 (45.2%)
The most frequent surgical indication was intestinal obstruction 62.9%. A majority of 61.1% were operated in emergency settings. Most frequent location of the disease was the small bowel (62.9% of the cases). Most of the surgical interventions were done via open technique 72.6% while the rest were operated via minimally invasive procedures (laparoscopy).
Smokers had surgery on a mean of 4 years before non-smokers (Table 1). Emergency surgery was required more frequently in the smoking group for intestinal obstruction P=0.02. Also, recurrence was diagnosed on a mean of 6 months faster in the smoking group than in the non-smoking group (Table 1). More interventions were done on the small bowel due to CD complications in the smoking group (P=0.029501) while the non-smoking shower a tendency to complications located on the colon (P=0.009796). Also, the smoking group required surgery more often (24.4%) vs. (17.6%). Recurrences in the non-smoking group responded better to medical therapy (Table 1).

Full table
Most patients were from an urban environment, they had a shorter interval to recurrence and were operated more frequently in emergency settings with n=31/43 (72.1%) compared to patients from rural environments, n=10/19 (52.6%). Only n=12/43 (27.9%) from the urban environment were operated in elective settings. Patients from urban environments were more frequently operated for life threating complications such as intestinal obstruction results P=0.004—n=7/19 (36.8%) vs. n=32/43 (74.4%). The recurrence-free interval favored the rural environments 2.05 years vs. urban environments 1.12 years. Taking all of this into consideration, postoperative remission was encountered more often in urban environments—but the results were not statistically relevant with a P=0.5.
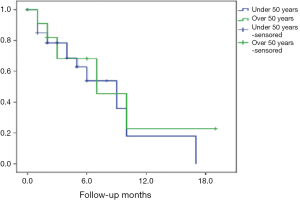
To assess whether age can influence postoperative recurrence, the group was dichotomized in a group with less than 50 years and a group over 50 years, and the results were represented through a Kaplan-Meier curve where the event is a surgical reintervention secondary to CD complications (Figure 1). There was no difference in the two subgroups—the curves overlap with P=0.15 (Fisher’s Exact Test). The choice of preop medical treatment was not influenced by age (Table 2). Patients aged less than 50 years experienced a more severe evolution of the disease with more frequent involvement of the small bowel and frequent surgery due to obstruction P=0.00004 (Table 2). The duration to recurrence was shorter in the group of patients under 50 years (35.7% vs. 27.1%). Maximum medical therapy failure is one of the operative indications in CD. This was the case of only 7% of the patients under 50 and 30% of patients older than 50. This demonstrates the aggressiveness of the disease in the younger age (Table 2). Recurrence did not show a tendency regarding a specific intestinal location when age was taken into account (Table 2).

Full table
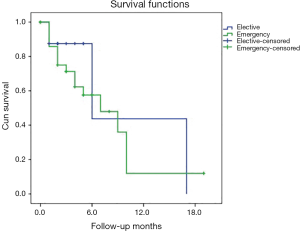
In order to assess whether the operative moment—emergency vs. elective may influence postoperative recurrence rate—the main group of patients was divided into two subgroups and compared and graphically represented by a Kaplan-Meier curve (Figure 2). Patients with elective surgery experienced a much more linear and slower progression towards recurrence than patients with emergency surgeries (Figure 2). The recurrence rate between the two groups, however, did not differ P=0.7 (Mantel-Cox test).
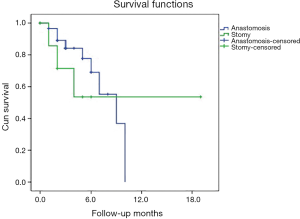
Using as a starting point the first surgical intervention which was associated with either anastomosis or stomy—the evolution of these patients was monitored over time —the anastomosis group was more frequently subjected to a surgical reintervention compared to the group that associated a stomy and had a slower progression to recurrence P=0.01 (Chi-Square test) (Figure 3).
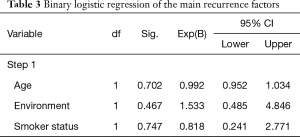
Statistical significance was lost after we performed a binary logistic regression. However, it was observed that as age increased the recurrence rate fell. Urban environment also correlated positively with the recurrence rate (Table 3). Smoking status did not predict a higher probability of recurrence, and this can be interpreted in context since the cohort consisted mainly of smokers (72%) (Table 3).
Full table
Discussion
Between 70 and 90% of patients with CD will require at least one surgical intervention during their lifetime (1). The probability of a patient to undergo surgery after the diagnosis of CD is 16.3% at 1 year, 33.3% at 3 years, and 46.6% at 5 years (1).
The surgical reintervention rate in CD is placed in the literature between 25–45% at 10 years after the first surgical procedure, data confirmed by our study group which places the surgical reintervention rate at 33% at 1.4 years from the first surgical operation. The duration from diagnosis to first surgery was 2.27 years as demonstrated in Figure 4 (1).
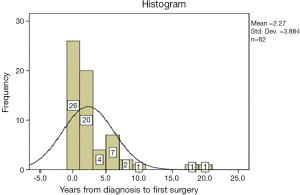
The assessment of the surgical risk of recurrence and reintervention presents some difficulties due to the small number of patients in the cohorts used for analysis and short follow-up periods—these combined elements have led to the impossibility to create and adopt a consensus which identifies recurrence risk predictors. A common problem with the analysis of follow-up data in patients diagnosed with CD and surgery is the lack of high-quality, prospective, randomized 1A studies. This issue has led to meta-analyses in which the long-term evolution of patients treated for CD complications are of doubtful quality. One of the reasons for this heterogeneity in results is due to the small number of patients which comprise the retrospective studies and to the multitude of variables that may influence the patient’s evolution (17).
It is disputable whether the genre of the patient can be considered a risk factor in patients with CD. Data from the literature is conflicting, Chardavoyne identified a higher incidence of recurrence in female patients, data confirmed by Lennard Jones et al. (17-20). The majority of studies presented by Sachar et al., Ellis et al., Caprilli et al., Rutgeerts et al. or Post et al. did not associate gender with the recurrence rate (21-25). There was a predominance of male patients in the reintervention subgroup—an explanation would be that the main group of patients was composed in the majority of males. Also, male patients usually have a lower rate of adherence to medical recommendations made by the medical staff. Thus the authors consider that an organic cause for the higher incidence in males is not likely, but further research on this subject is warranted.
The habit of smoking has begun to receive more attention regarding its role in CD genesis, and its an influence on postoperative recurrence rate (26). Data from the studied group confirms this trend that CD patients are also frequent smokers. After gender stratification, we did not find a higher incidence of female patients among the smokers group P=0.4 in contradiction with Sutherland et al. who identified a higher incidence in female smokers (Table 1) (27). Also, the literature suggests that heavy smokers >10 cigarettes/day also associate a higher risk of the first surgery and surgical reintervention (28). Unfortunately, due to the retrospective nature of the study, it was not possible to statistically assess this relationship between the quantity of tobacco consumed and the evolution of the disease. The smoking group had a smaller percentage of postoperative remissions and more aggressive recurrences which frequently required surgery than the non-smoking group (Table 1). These data are also confirmed by the literature (29). Smokers require at least one extra-surgical intervention when compared to non-smokers, data that we could not confirm. The surgical reintervention rate was similar in both groups but the smoking - the group had a shorter recurrence-free interval. Similar data regarding the lack of impact on the recurrence rate were also obtained by Medina et al. (29). However, it seems that smoking cessation positively affects both the CD incidence and evolution over time. Since the majority of patients are not aware of these associations of severity between CD and smoking, giving up tobacco through education and awareness, should be included as one of the possible treatments for CD, not a facultative option.
CD is recognized as having a major impact on industrialized nations. This assertion is rooted in the prevalence of CD, with high levels of incidence in Europe and the United States (30). In these regions CD patients derive mainly from urban environments (30). Several studies have investigated this association—but the results have been inconclusive. Establishing the fact that urban populations are more exposed to surgery and CD is a piece of important information as it can direct future research into CD while allowing resource allocation for its treatment and prophylaxis. The urbanization of society has become a risk factor in developing CD according to the systematic review published in 2012 by Soon et al. (31). Indeed, the indications for surgery in patients from urban environments were more severe in terms of diagnosis - so urban patients were more frequently operated for intestinal occlusion - statistically significant data with P=0.004. Life in the countryside seems to have a protective effect on CD, especially in the first 5 years of life—and this effect persists for the whole life of the individual (32). The mechanisms by which this protective effect is mitigated is not yet fully understood—a theory commonly discussed is the hygiene theory which corresponds to the fact that the child in early life in the urban environment is exposed to a limited number of pathogens and the immune system at the level of the digestive tract is not fully stimulated. In adult life when exposed to new pathogens, the individual develops an exaggerated reaction that also goes against its structures. Urban patients underwent emergency surgery more frequently than in rural areas, but it should be taken into account that they had faster access to specialized medical services. Complications such as an intestinal obstruction in this subgroup were more severe. The protective effect of the rural environment also materialized on the surgical recurrence-free interval—rural patients had a longer period to second surgery than the patients from urban areas.
By dichotomizing the group of patients using the age of 50 years as cut-off it was observed that in the group with patients >50 years, the most common co-morbidity was neoplasia. Data that can be confirmed by the literature—the incidence of neoplasms increased proportionally with age (33). In 1952 Rosenberg and Crohn demonstrated the link between colorectal cancer and CD—its incidence varying with the extent of the lessions, duration, and severity of colon inflammation. At present, CD accounts for 1–2% of all colorectal cancer. The risk increases by 1%/year after 8 years of evolution (33,34). Regarding the association of small bowel carcinoma with CD and increased age—data available for analysis is scarce. However, it is estimated that the risk of developing a small intestinal adenocarcinoma is 60× higher in patients suffering from this disease (35). Small bowel carcinoma is a pathology that is difficult to diagnose in this category of patients. It is usually identified only at the time of surgery for what was initially considered an exacerbation of the disease. In the studied group, we did not encounter CD patients with intestinal adenocarcinoma. The group of patients aged <50 years associated a higher rate of emergency surgery for intestinal occlusion and recurrence and were treated in large part by surgical interventions thus suggesting the aggressiveness of the disease in this subgroup (Table 2). In the group of patients >50 years, only one patient was treated by surgery—the rest of the cases received medical treatment when recurrence was encountered (Table 2). There are many theories about higher CD aggression in younger patients. They adhere less to the recommendations of hygienic—dietary regimens and the medical treatment. Another important aspect is the immune system—so the relative immunosuppression status and the slow reactivity of the immune system relates to a better response to medical therapy thus reducing both the incidence of flares and their intensity—sheltering the patient from possible recurrence and surgery (36,37). There was also a statistically significant prevalence of small bowel disease (P=0.000261) in younger patients compared to older patients who had colon disease predominantly (P=0.000041) (Table 2). This variation in location was most likely due to higher CD aggression in young patients with more frequent involvement of the small bowel. This correlation was also identified by Polito et al. (38). Duration to recurrence was slightly longer in the group of patients over 50 years, but the results did not show statistical significance. Regarding the therapeutic attitude in surgery, it appears that age did not play a decisive role in choosing an anastomosis or stomy, so 57% of patients over 50 years had an anastomosis while 73% of patients under the age of 50 years had an anastomosis done with a P value of 0.3. Regardless of the time of surgery: emergency vs. elective, the basic therapeutic option in both situations was resection with anastomosis. The recurrence rate was not influenced by the moment of surgery as observed in Figure 2. Age did not influence the recurrence rate—the Kaplan-Meier curves overlap while Fischer’s exact test was P=0.15 as demonstrated in Figure 1.
While technology has evolved, it offered the possibility to unlock the entire human genome, thus over 140 loci in genes that might be involved in CD pathogenesis or be used as prognostic markers for CD have been identified. An important role was attributed to NOD2/CARD15 genes located on chromosome 16, but also to the OCTN1 gene located on chromosome 5 and the DLG5 gene (LARGE DISC HOMOLOG 5) located on chromosome 10 in the genesis of CD (39-42). The exact mechanism by which NOD2/CARD15 gene mutation occurs and their role in mediating intestinal immune response remain unclear, but their mutations cause changes in the intestinal mucosal barrier and genesis/maintenance of the immune response (43,44).
Conclusions
The mean time from diagnosis to first surgery was 2.27 years so careful monitoring should be done postoperatively in patients with risk factors (young age, urban environment, smokers). Patients under 50 years old seem to have a more aggressive evolution of the disease regarding the intensity of the flares. Giving up smoking may increase the recurrence-free interval. Urban patients have a more severe evolution of CD in terms of diagnosis, surgical complications, and post-surgical evolution compared to rural patients.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/map.2019.03.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of the Emergency Clinical Hospital Sfanta Maria, Bucharest, Romania (No. 25649) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bednarz W, Czopnik P, Wojtczak B, et al. Analysis of results of surgical treatment in Crohn’ s disease. Hepatogastroenterology 2008;55:998-1001. [PubMed]
- Frolkis AD, Dykeman J, Negrón ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology 2013;145:996-1006. [Crossref] [PubMed]
- Williams C, Panaccione R, Ghosh S, et al. Optimizing clinical use of mesalazine (5-aminosalicylic acid) in inflammatory bowel disease. Therap Adv Gastroenterol 2011;4:237-48. [Crossref] [PubMed]
- Ardizzone S, Cassinotti A, Manes G, et al. Immunomodulators for all patients with inflammatory bowel disease? Therap Adv Gastroenterol 2010;3:31-42. [Crossref] [PubMed]
- Sandborn WJ, Targan SR. Biologic therapy of inflammatory bowel disease. Gastroenterology 2002;122:1592-608. [Crossref] [PubMed]
- Loftus EV Jr, Schoenfeld P, Sandborn WJ. The epidemiology and natural history of Crohn's disease in population-based patient cohorts from North America: a systematic review. Aliment Pharmacol Ther 2002;16:51-60. [Crossref] [PubMed]
- Goyer P, Alves A, Bretagnol F, et al. Impact of complex Crohn's disease on the outcome of laparoscopic ileocecal resection: a comparative clinical study in 1 patient. Dis Colon Rectum 2009;52:205-10. [Crossref] [PubMed]
- Hemstreet BA. Chapter 21. Inflammatory Bowel Disease. In: Di Piro JT, Talbert RL, Yee GC, et al. editors. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York: McGraw-Hill, 2014.
- Abegunde AT, Muhammad BH, Ali T. Preventive health measures in inflammatory bowel disease. World J Gastroenterol 2016;22:7625-44. [Crossref] [PubMed]
- Nos P, Domènech E. Management of Crohn's disease in smokers: is an alternative approach necessary? World J Gastroenterol 2011;17:3567-74. [Crossref] [PubMed]
- Cheifetz AS. Management of active Crohn disease. JAMA 2013;309:2150-8. [Crossref] [PubMed]
- Haneda S, Ohnuma S, Musha H, et al. Outcome of surgical treatment for Crohn's disease. Eur Surg Res 2014;52:195-6.
- Kono E, Haneda S, Ohnuma S, et al. Long-term outcome of intestinal failure requiring home parenteral nutrition in patients with Crohn's disease. Eur Surg Res 2014;52:220-1.
- Shaffer VO, Wexner SD. Surgical management of Crohn’s disease. Langenbecks Arch Surg 2013;398:13-27. [Crossref] [PubMed]
- Cottone M, Rosselli M, Orlando A. Smoking habits and recurrence in Crohn’s disease. Gastroenterology 1994;106:643-8. [Crossref] [PubMed]
- Wettergren A, Christianson J. Risk of recurrence and reoperation after resection for ileocolic Crohn's disease. Scand J Gastroenterol 1991;26:1319-22. [Crossref] [PubMed]
- Heimann TM, Greenstein AJ, Lewis B, et al. Comparison of primary and reoperative surgery in patients with Crohns disease. Ann Surg 1998;227:492-5. [Crossref] [PubMed]
- Delaney J, Laws P, Wille-Jørgensen P, et al. Inflammatory bowel disease meta-evidence and its challenges: is it time to restructure surgical research? Colorectal Dis 2015;17:600-11. [Crossref] [PubMed]
- Lennard Jones JE, Stalder GA. Prognosis after resection of chronic regional ileitis. Gut 1971;8:332-6. [Crossref] [PubMed]
- Chardavoyne R, Flint GW, Pollack S, et al. Factors affecting recurrence following resection for Crohn’s disease. Dis Colon Rectum 1986;29:495-502. [Crossref] [PubMed]
- Sachar DB, Wolfson DM, Greenstein AJ, et al. Risk factors for postoperative recurrence of Crohn’s disease. Gastroenterology 1983;85:917-21. [Crossref] [PubMed]
- Ellis L, Calhoun P, Kaiser DL, et al. Postoperative recurrence in Crohn’s disease: the effect of the initial length of bowel resection. Ann Surg 1984;199:340-7. [PubMed]
- Caprilli R, Corrao G, Taddei G, et al. Gruppo Italiano per lo Studio del Colon e del Retto (GISC). Prognostic factors for postoperative recurrence of Crohn’s disease. Dis Colon Rectum 1996;39:335-41. [Crossref] [PubMed]
- Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990;99:956-63. [Crossref] [PubMed]
- Post S, Herfarth C, Bohm E, et al. The impact of disease pattern, surgical management, and individual surgeons on the risk for relaparotomy for recurrent Crohn’s disease. Ann Surg 1996;223:253-60. [Crossref] [PubMed]
- Ryan WR, Allan RN, Yamamoto T, et al. Crohn’s disease patients who quit smoking have a reduced risk of reoperation for recurrence. Am J Surg 2004;187:219-25. [Crossref] [PubMed]
- Sutherland LR, Ramcharan S, Bryant H, et al. Effects of cigarette smoking on recurrence of Crohn’s disease. Gastroenterology 1990;98:1123-8. [Crossref] [PubMed]
- Yamamoto T, Keighley MR. The association of cigarette smoking with a high risk of recurrence after ileocolonic resection for ileocecal Crohn’s disease. Surg Today 1999;29:579-80. [Crossref] [PubMed]
- Medina C, Vergara M, Casellas F, et al. Influence of the smoking habit in the surgery of inflammatory bowel disease. Rev Esp Enferm Dig 1998;90:771-8. [PubMed]
- Bernstein CN, Blanchard JF, Rawsthorne P, et al. Epidemiology of Crohn's disease and ulcerative colitis in a central Canadian province: a population-based study. Am J Epidemiol 1999;149:916-24. [Crossref] [PubMed]
- Soon IS, Molodecky NA, Rabi DM, et al. The relationship between urban environment and the inflammatory bowel diseases: a systematic review and meta-analysis. BMC Gastroenterol 2012;12:51. [Crossref] [PubMed]
- Benchimol EI, Kaplan GG, Otley AR, et al. Rural and Urban Residence During Early Life is Associated with Risk of Inflammatory Bowel Disease: A Population-Based Inception and Birth Cohort Study. Am J Gastroenterol 2017;112:1412-22. [Crossref] [PubMed]
- Crohn BB, Rosenberg H. The sigmoidoscopic picture of chronic ulcerative colitis. Am J Med Sci 1925;170:220-8. [Crossref]
- Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001;48:526-35. [Crossref] [PubMed]
- Jess T, Winther KV, Munkholm P, et al. Intestinal and extra-intestinal cancer in Crohn's disease: follow-up of a population-based cohort in Copenhagen County, Denmark. Aliment Pharmacol Ther 2004;19:287-93. [Crossref] [PubMed]
- Horne R, Parham R, Driscoll R, et al. Patients' attitudes to medicines and adherence to maintenance treatment in inflammatory bowel disease. Inflamm Bowel Dis 2009;15:837-44. [Crossref] [PubMed]
- Dorshkind K, Montecino-Rodriguez E, Signer RA. The ageing immune system: is it ever too old to become young again? Nat Rev Immunol 2009;9:57-62. [Crossref] [PubMed]
- Polito JM 2nd, Childs B, Mellits ED, et al. Crohn's disease: influence of age at diagnosis on site and clinical type of disease. Gastroenterology 1996;111:580-6. [Crossref] [PubMed]
- Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 2001;411:603-6. [Crossref] [PubMed]
- Hampe J, Cuthbert A, Croucher PJ, et al. Association between insertion mutation in NOD2 gene and Crohn’s disease in German and British populations. Lancet 2001;357:1925-8. [Crossref] [PubMed]
- Armuzzi A, Ahmad T, Ling KL, et al. Genotype- phenotype analysis of the Crohn’s disease susceptibility haplotype on chromosome 5q31. Gut 2003;52:1133-9. [Crossref] [PubMed]
- Peltekova VD, Wintle RF, Rubin LA, et al. Functional variants of OCTN cation transporter genes are associated with Crohn disease. Nat Genet 2004;36:471-5. [Crossref] [PubMed]
- Rosenstiel P, Fantini M, Brautigam K, et al. TNF-alpha and IFN-gamma regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology 2003;124:1001-9. [Crossref] [PubMed]
- Hisamatsu T, Suzuki M, Reinecker HC, et al. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology 2003;124:993-1000. [Crossref] [PubMed]
Cite this article as: Slavu I, Tulin A, Alecu L, Birla R, Constantinoiu S. Post-surgical stratification of recurrence predictors for Crohn disease patients. Mesentery Peritoneum 2021;5:1.


